Calcium »
PDB 1b85-1bjj »
1b8l »
Calcium in PDB 1b8l: Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin
Protein crystallography data
The structure of Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin, PDB code: 1b8l
was solved by
M.S.Cates,
M.B.Berry,
E.Ho,
Q.Li,
J.D.Potter,
G.N.Phillips Jr.,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 1.70 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 34.511, 37.298, 37.067, 90.00, 113.12, 90.00 |
| R / Rfree (%) | 16.8 / 22.7 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin
(pdb code 1b8l). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total only one binding site of Calcium was determined in the Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin, PDB code: 1b8l:
In total only one binding site of Calcium was determined in the Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin, PDB code: 1b8l:
Calcium binding site 1 out of 1 in 1b8l
Go back to
Calcium binding site 1 out
of 1 in the Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin
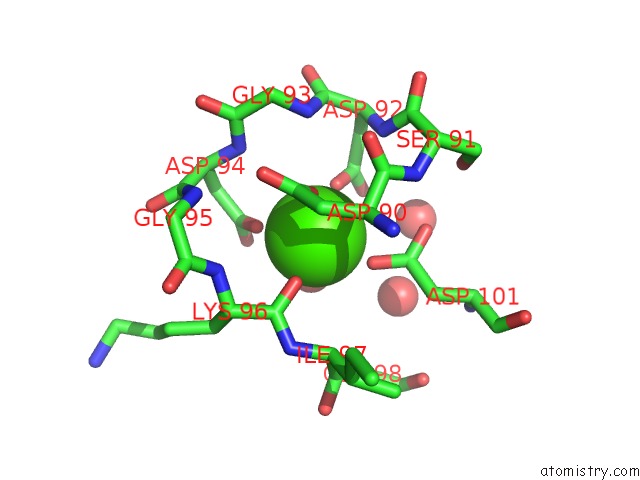
Mono view
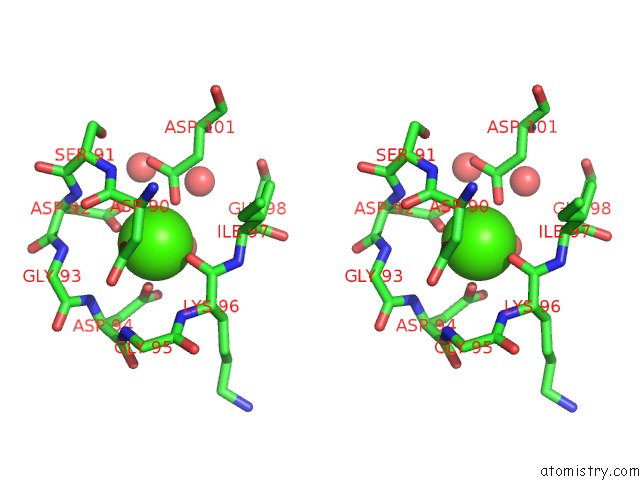
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin within 5.0Å range:
|
Reference:
M.S.Cates,
M.B.Berry,
E.L.Ho,
Q.Li,
J.D.Potter,
G.N.Phillips Jr..
Metal-Ion Affinity and Specificity in Ef-Hand Proteins: Coordination Geometry and Domain Plasticity in Parvalbumin. Structure Fold.Des. V. 7 1269 1999.
ISSN: ISSN 0969-2126
PubMed: 10545326
DOI: 10.1016/S0969-2126(00)80060-X
Page generated: Mon Jul 7 13:36:48 2025
ISSN: ISSN 0969-2126
PubMed: 10545326
DOI: 10.1016/S0969-2126(00)80060-X
Last articles
Cl in 5G54Cl in 5G4A
Cl in 5G4Q
Cl in 5G47
Cl in 5G42
Cl in 5G3S
Cl in 5G2P
Cl in 5G2T
Cl in 5G36
Cl in 5G2D