Calcium »
PDB 1krf-1l3f »
1l3f »
Calcium in PDB 1l3f: Thermolysin in the Absence of Substrate Has An Open Conformation
Enzymatic activity of Thermolysin in the Absence of Substrate Has An Open Conformation
All present enzymatic activity of Thermolysin in the Absence of Substrate Has An Open Conformation:
3.4.24.27;
3.4.24.27;
Protein crystallography data
The structure of Thermolysin in the Absence of Substrate Has An Open Conformation, PDB code: 1l3f
was solved by
A.C.Hausrath,
B.W.Matthews,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.00 / 2.30 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 97.052, 97.052, 106.583, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.2 / 30.2 |
Other elements in 1l3f:
The structure of Thermolysin in the Absence of Substrate Has An Open Conformation also contains other interesting chemical elements:
| Zinc | (Zn) | 3 atoms |
Calcium Binding Sites:
The binding sites of Calcium atom in the Thermolysin in the Absence of Substrate Has An Open Conformation
(pdb code 1l3f). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 4 binding sites of Calcium where determined in the Thermolysin in the Absence of Substrate Has An Open Conformation, PDB code: 1l3f:
Jump to Calcium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Calcium where determined in the Thermolysin in the Absence of Substrate Has An Open Conformation, PDB code: 1l3f:
Jump to Calcium binding site number: 1; 2; 3; 4;
Calcium binding site 1 out of 4 in 1l3f
Go back to
Calcium binding site 1 out
of 4 in the Thermolysin in the Absence of Substrate Has An Open Conformation
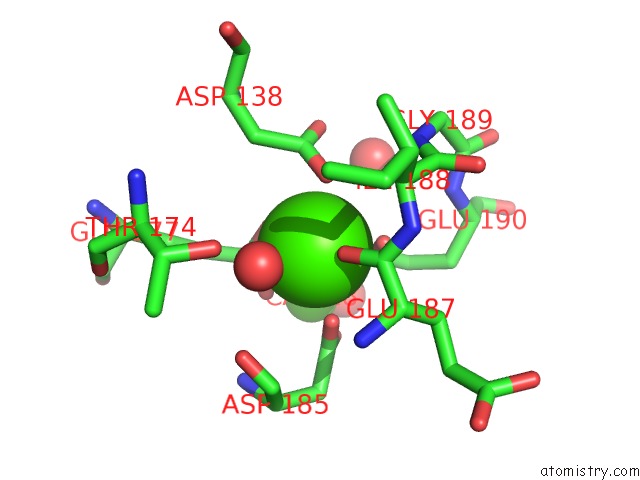
Mono view
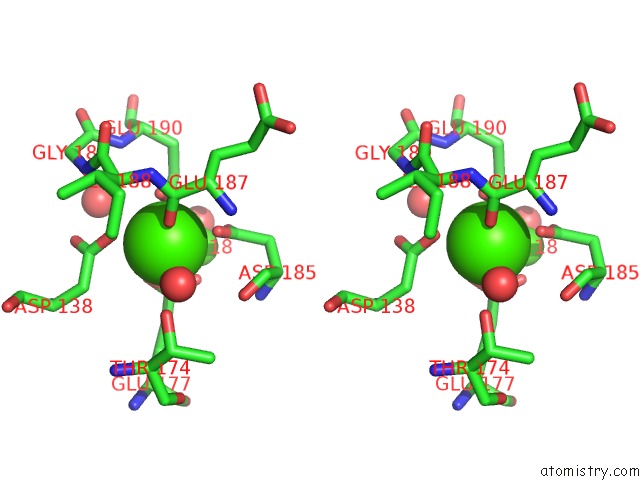
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Thermolysin in the Absence of Substrate Has An Open Conformation within 5.0Å range:
|
Calcium binding site 2 out of 4 in 1l3f
Go back to
Calcium binding site 2 out
of 4 in the Thermolysin in the Absence of Substrate Has An Open Conformation
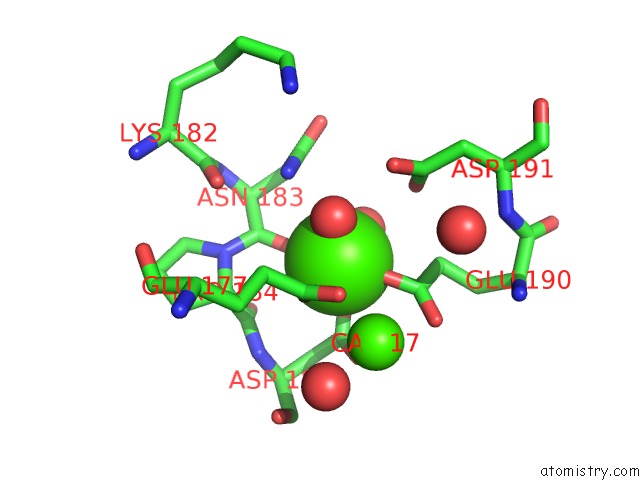
Mono view
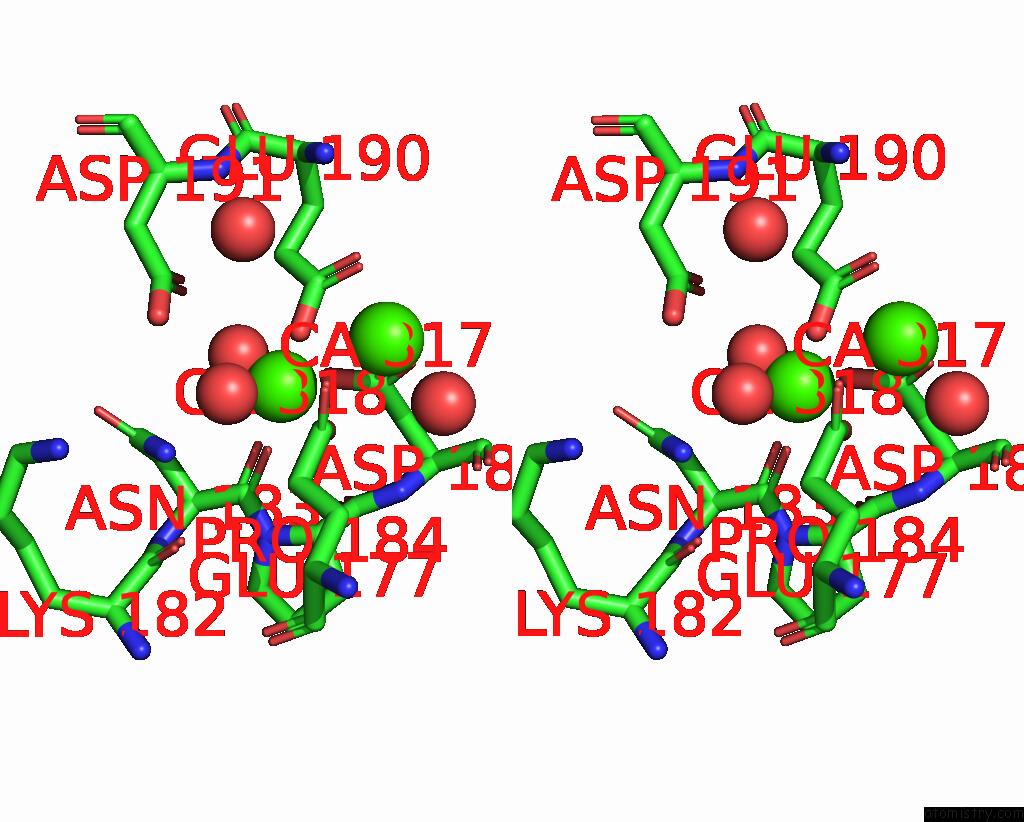
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Thermolysin in the Absence of Substrate Has An Open Conformation within 5.0Å range:
|
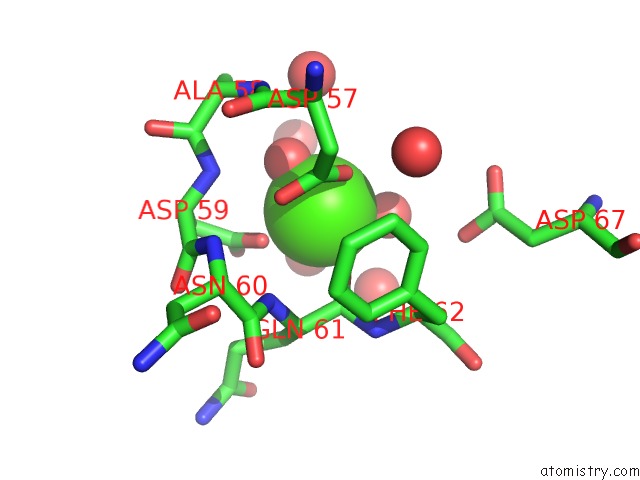
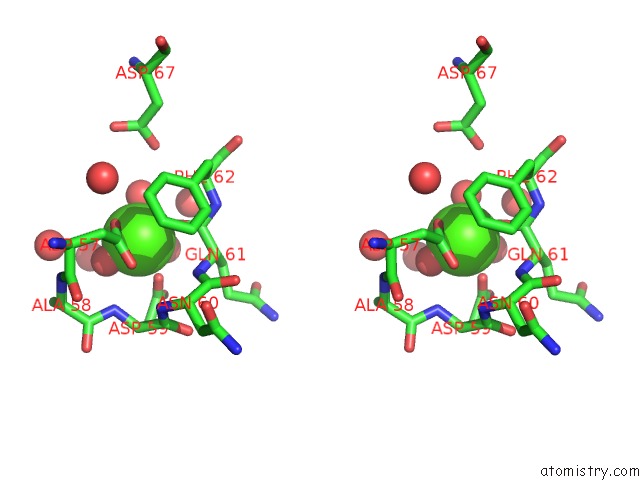
Calcium binding site 3 out of 4 in 1l3f
Go back to
Calcium binding site 3 out
of 4 in the Thermolysin in the Absence of Substrate Has An Open Conformation
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Thermolysin in the Absence of Substrate Has An Open Conformation within 5.0Å range:
|
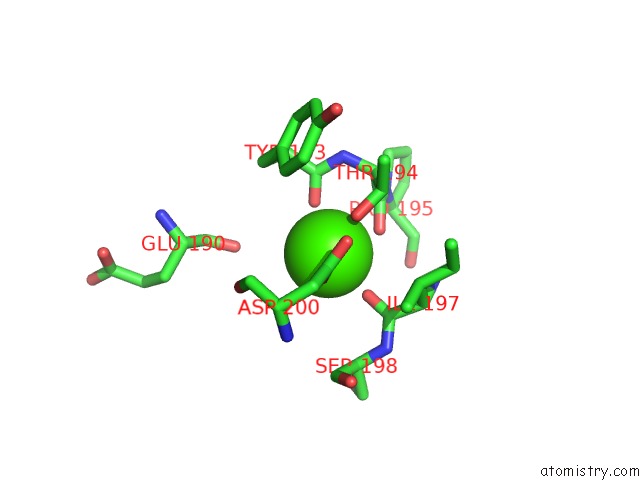
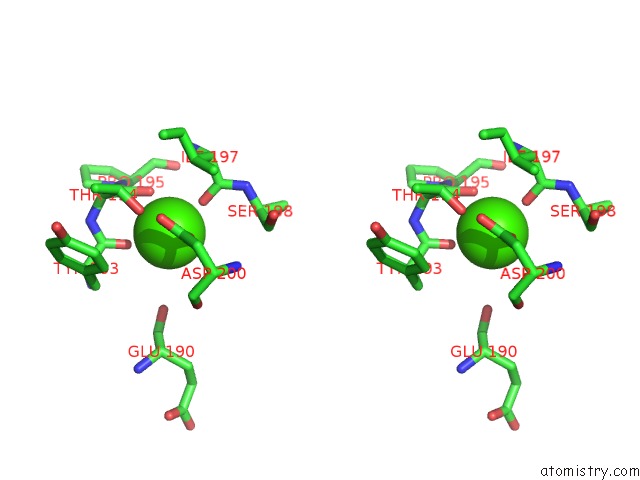
Calcium binding site 4 out of 4 in 1l3f
Go back to
Calcium binding site 4 out
of 4 in the Thermolysin in the Absence of Substrate Has An Open Conformation
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 4 of Thermolysin in the Absence of Substrate Has An Open Conformation within 5.0Å range:
|
Reference:
A.C.Hausrath,
B.W.Matthews.
Thermolysin in the Absence of Substrate Has An Open Conformation. Acta Crystallogr.,Sect.D V. 58 1002 2002.
ISSN: ISSN 0907-4449
PubMed: 12037302
DOI: 10.1107/S090744490200584X
Page generated: Mon Jul 7 16:45:43 2025
ISSN: ISSN 0907-4449
PubMed: 12037302
DOI: 10.1107/S090744490200584X
Last articles
Cl in 5JX3Cl in 5JXI
Cl in 5JXH
Cl in 5JXG
Cl in 5JWM
Cl in 5JWU
Cl in 5JWS
Cl in 5JWW
Cl in 5JVH
Cl in 5JWI