Calcium »
PDB 1ukg-1ux6 »
1uks »
Calcium in PDB 1uks: Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose
Enzymatic activity of Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose
All present enzymatic activity of Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose:
2.4.1.19;
2.4.1.19;
Protein crystallography data
The structure of Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose, PDB code: 1uks
was solved by
K.Haga,
R.Kanai,
O.Sakamoto,
K.Harata,
K.Yamane,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 1.90 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 64.850, 74.530, 78.940, 85.19, 104.86, 101.04 |
| R / Rfree (%) | 16.2 / 21.5 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose
(pdb code 1uks). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 4 binding sites of Calcium where determined in the Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose, PDB code: 1uks:
Jump to Calcium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Calcium where determined in the Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose, PDB code: 1uks:
Jump to Calcium binding site number: 1; 2; 3; 4;
Calcium binding site 1 out of 4 in 1uks
Go back to
Calcium binding site 1 out
of 4 in the Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose
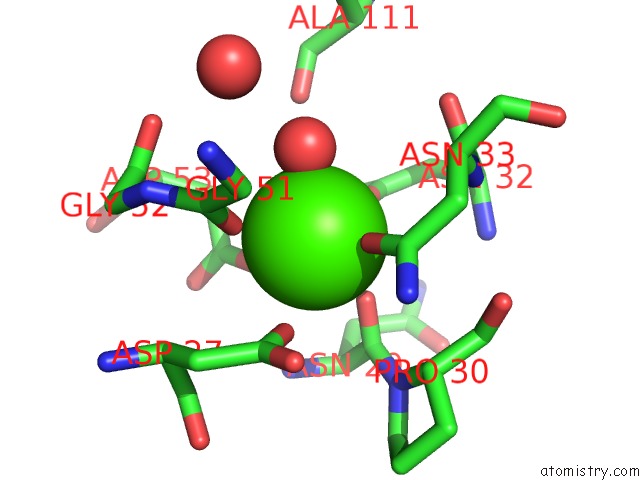
Mono view
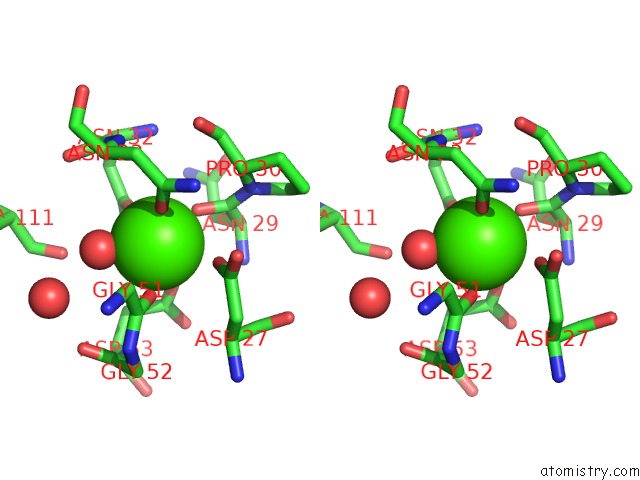
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose within 5.0Å range:
|
Calcium binding site 2 out of 4 in 1uks
Go back to
Calcium binding site 2 out
of 4 in the Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose
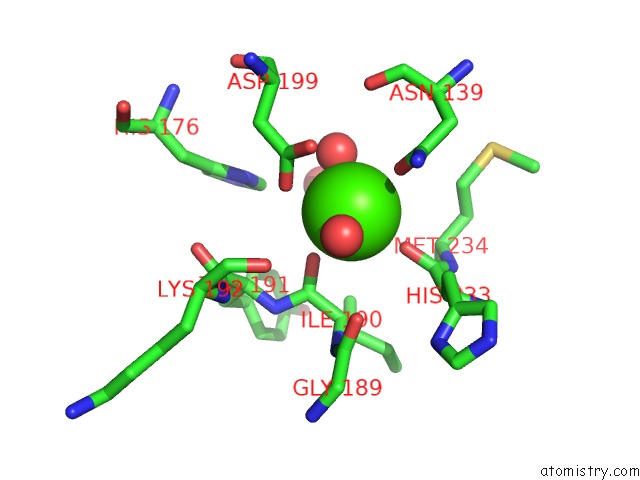
Mono view
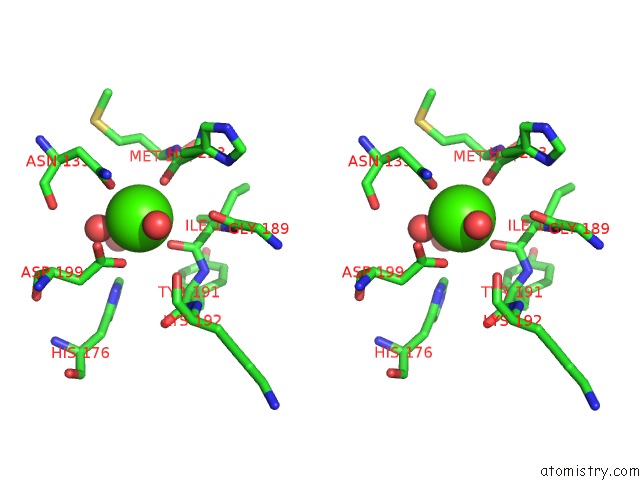
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose within 5.0Å range:
|
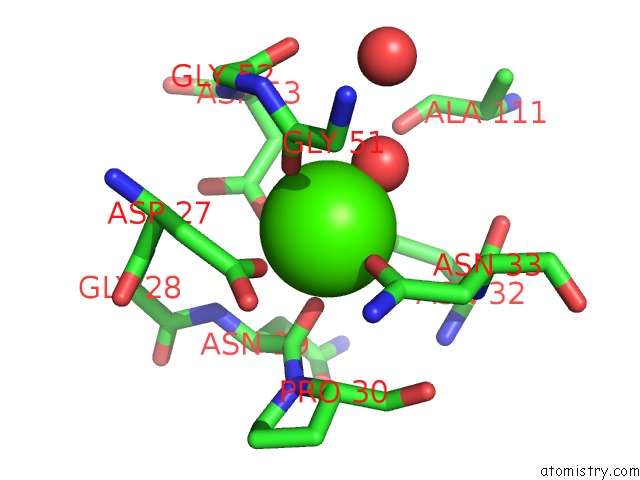
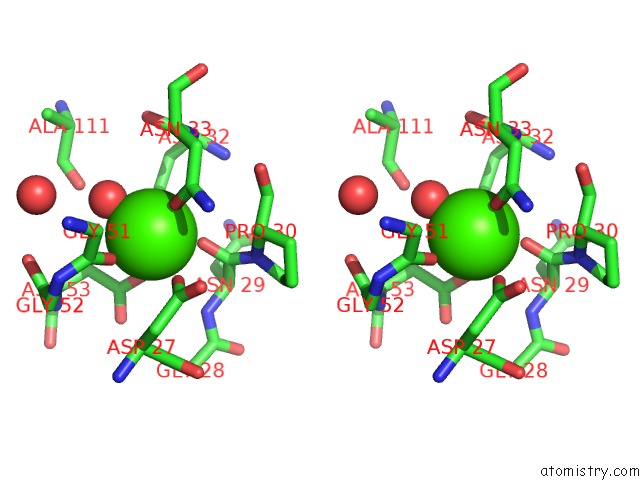
Calcium binding site 3 out of 4 in 1uks
Go back to
Calcium binding site 3 out
of 4 in the Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose within 5.0Å range:
|
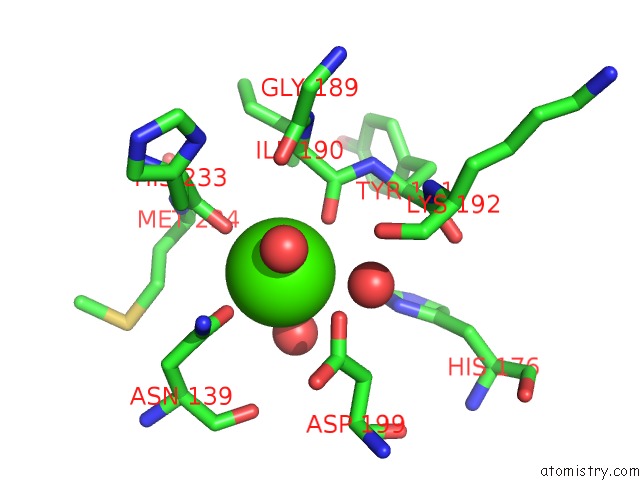
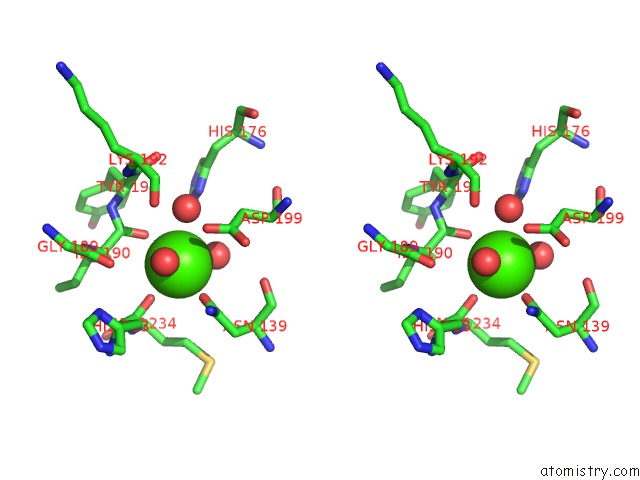
Calcium binding site 4 out of 4 in 1uks
Go back to
Calcium binding site 4 out
of 4 in the Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 4 of Crystal Structure of F183L/F259L Mutant Cyclodextrin Glucanotransferase Complexed with A Pseudo-Maltotetraose Derived From Acarbose within 5.0Å range:
|
Reference:
K.Haga,
R.Kanai,
O.Sakamoto,
M.Aoyagi,
K.Harata,
K.Yamane.
Effects of Essential Carbohydrate/Aromatic Stacking Interaction with TYR100 and PHE259 on Substrate Binding of Cyclodextrin Glycosyltransferase From Alkalophilic Bacillus Sp. 1011 J.Biochem.(Tokyo) V. 134 881 2003.
ISSN: ISSN 0021-924X
PubMed: 14769878
DOI: 10.1093/JB/MVG215
Page generated: Tue Jul 8 02:35:14 2025
ISSN: ISSN 0021-924X
PubMed: 14769878
DOI: 10.1093/JB/MVG215
Last articles
Cl in 5JJMCl in 5JIZ
Cl in 5JJ5
Cl in 5JHR
Cl in 5JIU
Cl in 5JHS
Cl in 5JGV
Cl in 5JHB
Cl in 5JHA
Cl in 5JH8