Calcium »
PDB 1ukg-1ux6 »
1umt »
Calcium in PDB 1umt: Stromelysin-1 Catalytic Domain with Hydrophobic Inhibitor Bound, pH 7.0, 32OC, 20 Mm CACL2, 15% Acetonitrile; uc(Nmr) Average of 20 Structures Minimized with Restraints
Enzymatic activity of Stromelysin-1 Catalytic Domain with Hydrophobic Inhibitor Bound, pH 7.0, 32OC, 20 Mm CACL2, 15% Acetonitrile; uc(Nmr) Average of 20 Structures Minimized with Restraints
All present enzymatic activity of Stromelysin-1 Catalytic Domain with Hydrophobic Inhibitor Bound, pH 7.0, 32OC, 20 Mm CACL2, 15% Acetonitrile; uc(Nmr) Average of 20 Structures Minimized with Restraints:
3.4.24.17;
3.4.24.17;
Other elements in 1umt:
The structure of Stromelysin-1 Catalytic Domain with Hydrophobic Inhibitor Bound, pH 7.0, 32OC, 20 Mm CACL2, 15% Acetonitrile; uc(Nmr) Average of 20 Structures Minimized with Restraints also contains other interesting chemical elements:
| Zinc | (Zn) | 2 atoms |
Calcium Binding Sites:
The binding sites of Calcium atom in the Stromelysin-1 Catalytic Domain with Hydrophobic Inhibitor Bound, pH 7.0, 32OC, 20 Mm CACL2, 15% Acetonitrile; uc(Nmr) Average of 20 Structures Minimized with Restraints
(pdb code 1umt). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total only one binding site of Calcium was determined in the Stromelysin-1 Catalytic Domain with Hydrophobic Inhibitor Bound, pH 7.0, 32OC, 20 Mm CACL2, 15% Acetonitrile; uc(Nmr) Average of 20 Structures Minimized with Restraints, PDB code: 1umt:
In total only one binding site of Calcium was determined in the Stromelysin-1 Catalytic Domain with Hydrophobic Inhibitor Bound, pH 7.0, 32OC, 20 Mm CACL2, 15% Acetonitrile; uc(Nmr) Average of 20 Structures Minimized with Restraints, PDB code: 1umt:
Calcium binding site 1 out of 1 in 1umt
Go back to
Calcium binding site 1 out
of 1 in the Stromelysin-1 Catalytic Domain with Hydrophobic Inhibitor Bound, pH 7.0, 32OC, 20 Mm CACL2, 15% Acetonitrile; uc(Nmr) Average of 20 Structures Minimized with Restraints
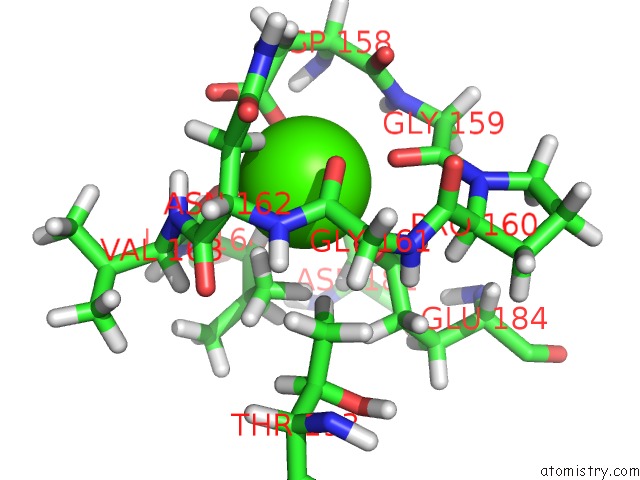
Mono view
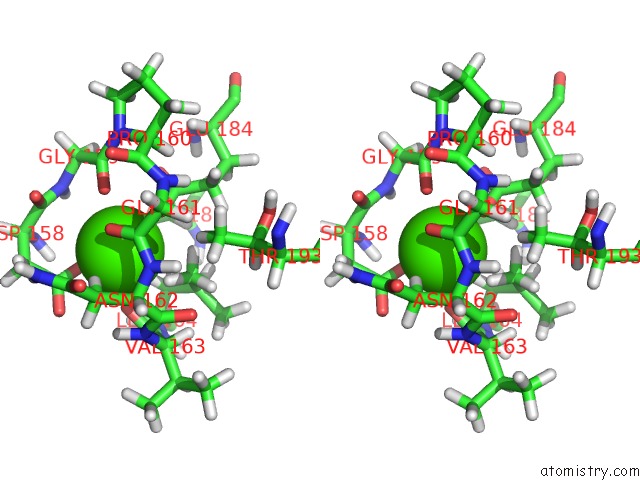
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Stromelysin-1 Catalytic Domain with Hydrophobic Inhibitor Bound, pH 7.0, 32OC, 20 Mm CACL2, 15% Acetonitrile; uc(Nmr) Average of 20 Structures Minimized with Restraints within 5.0Å range:
|
Reference:
S.R.Van Doren,
A.V.Kurochkin,
W.Hu,
Q.Z.Ye,
L.L.Johnson,
D.J.Hupe,
E.R.Zuiderweg.
Solution Structure of the Catalytic Domain of Human Stromelysin Complexed with A Hydrophobic Inhibitor. Protein Sci. V. 4 2487 1995.
ISSN: ISSN 0961-8368
PubMed: 8580839
Page generated: Thu Jul 11 23:32:50 2024
ISSN: ISSN 0961-8368
PubMed: 8580839
Last articles
Zn in 9MJ5Zn in 9HNW
Zn in 9G0L
Zn in 9FNE
Zn in 9DZN
Zn in 9E0I
Zn in 9D32
Zn in 9DAK
Zn in 8ZXC
Zn in 8ZUF