Calcium »
PDB 1w7x-1wws »
1wmd »
Calcium in PDB 1wmd: Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K)
Protein crystallography data
The structure of Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K), PDB code: 1wmd
was solved by
T.Nonaka,
M.Fujihashi,
A.Kita,
K.Saeki,
S.Ito,
K.Horikoshi,
K.Miki,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 100.00 / 1.30 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 42.840, 109.036, 166.912, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 13.4 / 16.9 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K)
(pdb code 1wmd). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 3 binding sites of Calcium where determined in the Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K), PDB code: 1wmd:
Jump to Calcium binding site number: 1; 2; 3;
In total 3 binding sites of Calcium where determined in the Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K), PDB code: 1wmd:
Jump to Calcium binding site number: 1; 2; 3;
Calcium binding site 1 out of 3 in 1wmd
Go back to
Calcium binding site 1 out
of 3 in the Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K)
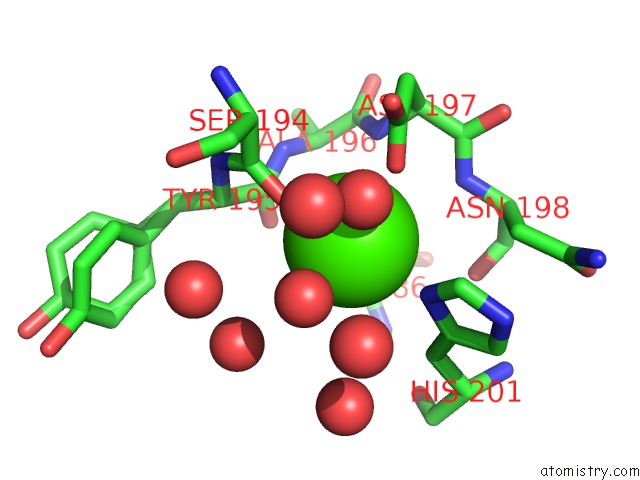
Mono view
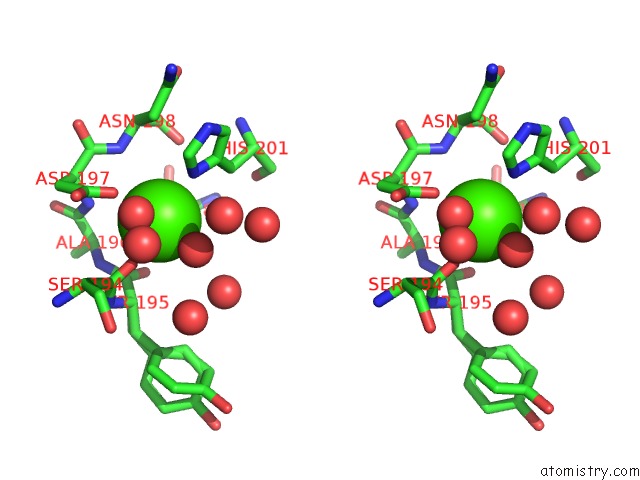
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K) within 5.0Å range:
|
Calcium binding site 2 out of 3 in 1wmd
Go back to
Calcium binding site 2 out
of 3 in the Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K)
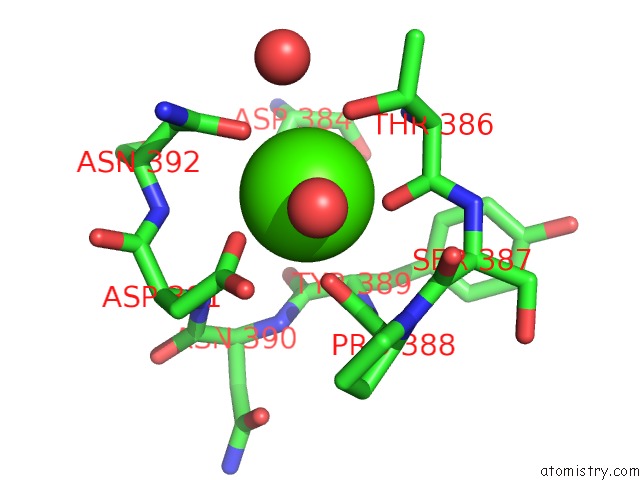
Mono view
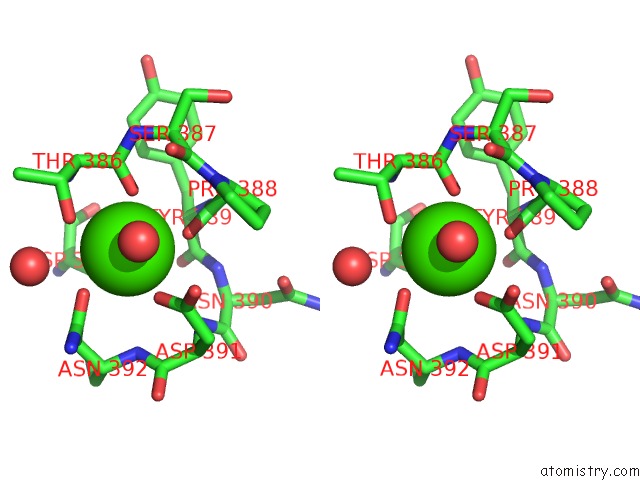
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K) within 5.0Å range:
|
Calcium binding site 3 out of 3 in 1wmd
Go back to
Calcium binding site 3 out
of 3 in the Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K)
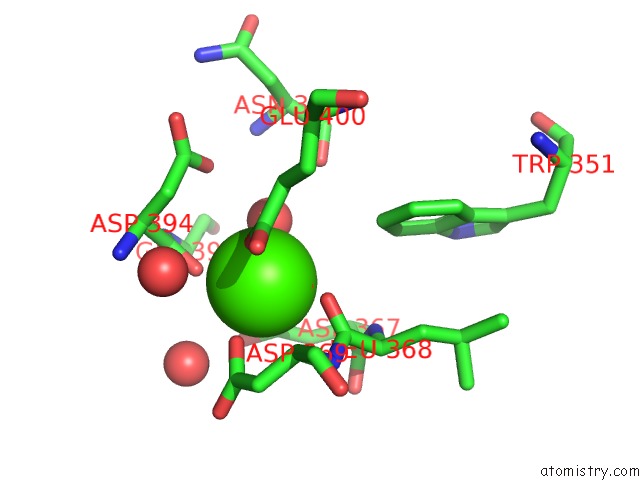
Mono view
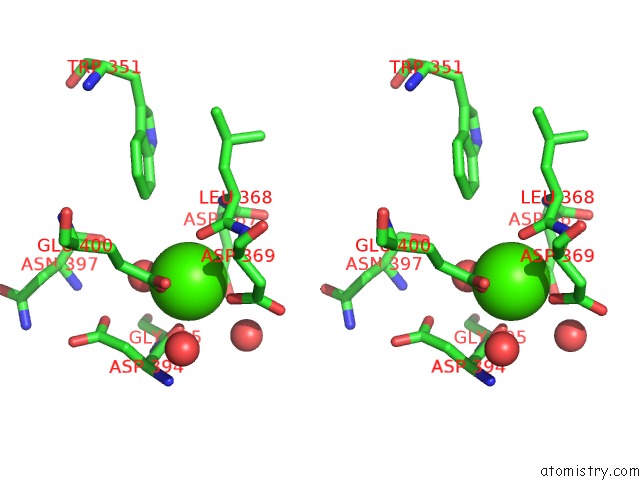
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Crystal Structure of Alkaline Serine Protease Kp-43 From Bacillus Sp. Ksm-KP43 (1.30 Angstrom, 100 K) within 5.0Å range:
|
Reference:
T.Nonaka,
M.Fujihashi,
A.Kita,
K.Saeki,
S.Ito,
K.Horikoshi,
K.Miki.
The Crystal Structure of An Oxidatively Stable Subtilisin-Like Alkaline Serine Protease, Kp-43, with A C-Terminal {Beta}-Barrel Domain J.Biol.Chem. V. 279 47344 2004.
ISSN: ISSN 0021-9258
PubMed: 15342641
DOI: 10.1074/JBC.M409089200
Page generated: Tue Jul 8 03:13:39 2025
ISSN: ISSN 0021-9258
PubMed: 15342641
DOI: 10.1074/JBC.M409089200
Last articles
Cl in 5HZXCl in 5I0R
Cl in 5I02
Cl in 5I00
Cl in 5HZ5
Cl in 5HZ8
Cl in 5HZD
Cl in 5HZ6
Cl in 5HY5
Cl in 5HXZ