Calcium »
PDB 1yf4-1yvn »
1yja »
Calcium in PDB 1yja: Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide
Enzymatic activity of Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide
All present enzymatic activity of Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide:
3.4.21.14;
3.4.21.14;
Protein crystallography data
The structure of Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide, PDB code: 1yja
was solved by
R.D.Kidd,
G.K.Farber,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 17.00 / 1.80 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 41.570, 79.670, 37.220, 90.00, 114.13, 90.00 |
| R / Rfree (%) | 21.5 / n/a |
Calcium Binding Sites:
The binding sites of Calcium atom in the Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide
(pdb code 1yja). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 2 binding sites of Calcium where determined in the Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide, PDB code: 1yja:
Jump to Calcium binding site number: 1; 2;
In total 2 binding sites of Calcium where determined in the Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide, PDB code: 1yja:
Jump to Calcium binding site number: 1; 2;
Calcium binding site 1 out of 2 in 1yja
Go back to
Calcium binding site 1 out
of 2 in the Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide
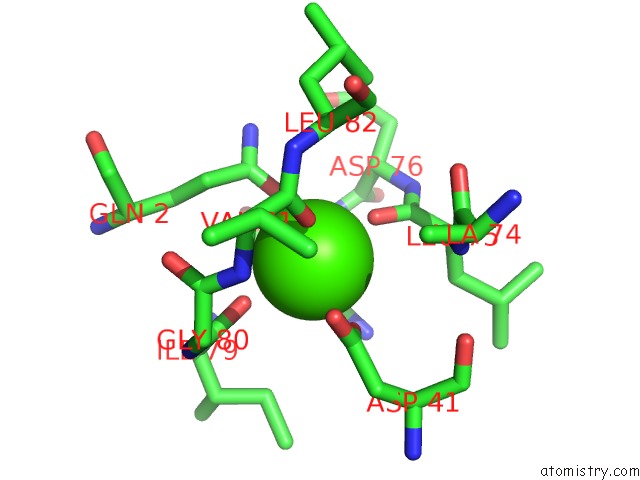
Mono view
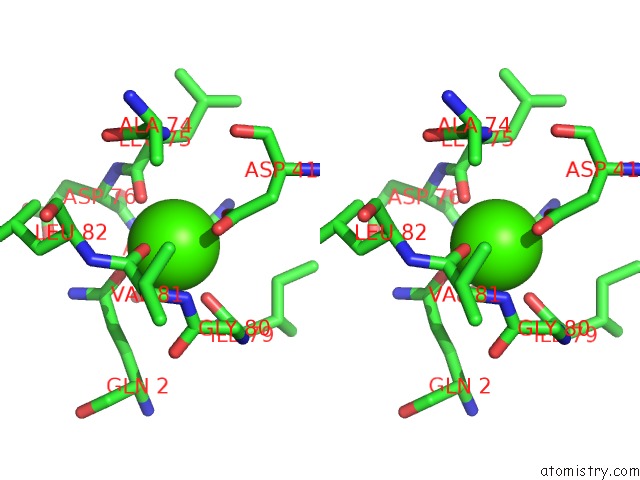
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide within 5.0Å range:
|
Calcium binding site 2 out of 2 in 1yja
Go back to
Calcium binding site 2 out
of 2 in the Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide
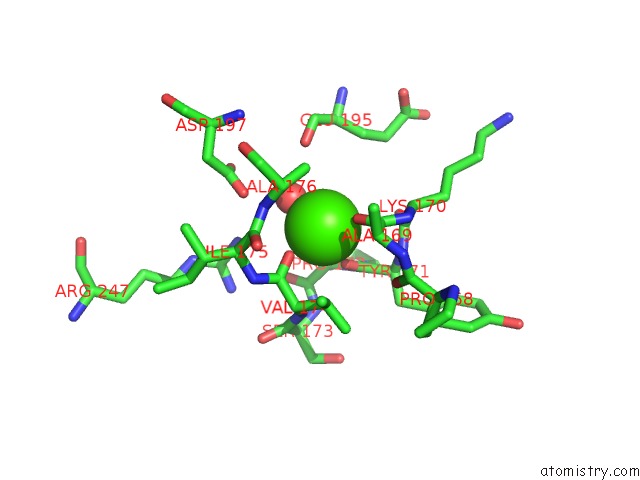
Mono view
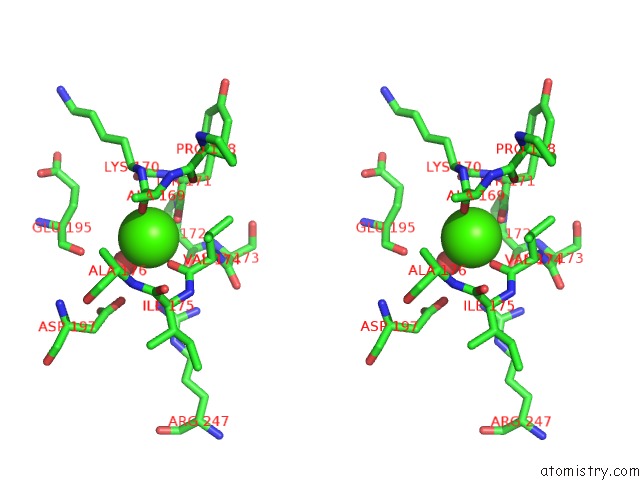
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Subtilisin Bpn' 8397+1 (E.C. 3.4.21.14) (Mutant with Met 50 Replaced By Phe, Asn 76 Replaced By Asp, Gly 169 Replaced By Ala, Gln 206 Replaced By Cys, Asn 218 Replaced By Ser and Lys 256 Replaced By Tyr) (M50F, N76D, G169A, Q206C, N218S, and K256Y) in 20% Dimethylformamide within 5.0Å range:
|
Reference:
R.D.Kidd,
P.Sears,
D.H.Huang,
K.Witte,
C.H.Wong,
G.K.Farber.
Breaking the Low Barrier Hydrogen Bond in A Serine Protease. Protein Sci. V. 8 410 1999.
ISSN: ISSN 0961-8368
PubMed: 10048334
Page generated: Tue Jul 8 03:51:53 2025
ISSN: ISSN 0961-8368
PubMed: 10048334
Last articles
F in 7Q85F in 7Q7R
F in 7Q7W
F in 7Q7L
F in 7PVA
F in 7Q6T
F in 7Q4A
F in 7PV7
F in 7Q2Y
F in 7Q3B