Calcium »
PDB 1zf0-2a2z »
1zmr »
Calcium in PDB 1zmr: Crystal Structure of the E. Coli Phosphoglycerate Kinase
Enzymatic activity of Crystal Structure of the E. Coli Phosphoglycerate Kinase
All present enzymatic activity of Crystal Structure of the E. Coli Phosphoglycerate Kinase:
2.7.2.3;
2.7.2.3;
Protein crystallography data
The structure of Crystal Structure of the E. Coli Phosphoglycerate Kinase, PDB code: 1zmr
was solved by
S.Marqusee,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 20.00 / 2.40 |
| Space group | P 32 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 77.887, 77.887, 195.276, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 21.9 / 25.3 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of the E. Coli Phosphoglycerate Kinase
(pdb code 1zmr). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 3 binding sites of Calcium where determined in the Crystal Structure of the E. Coli Phosphoglycerate Kinase, PDB code: 1zmr:
Jump to Calcium binding site number: 1; 2; 3;
In total 3 binding sites of Calcium where determined in the Crystal Structure of the E. Coli Phosphoglycerate Kinase, PDB code: 1zmr:
Jump to Calcium binding site number: 1; 2; 3;
Calcium binding site 1 out of 3 in 1zmr
Go back to
Calcium binding site 1 out
of 3 in the Crystal Structure of the E. Coli Phosphoglycerate Kinase
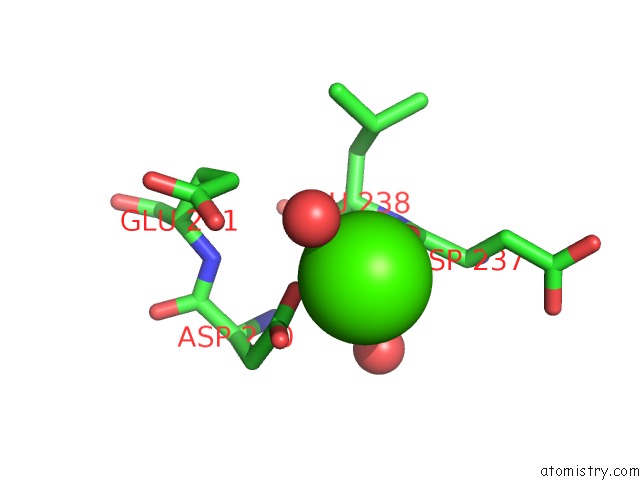
Mono view
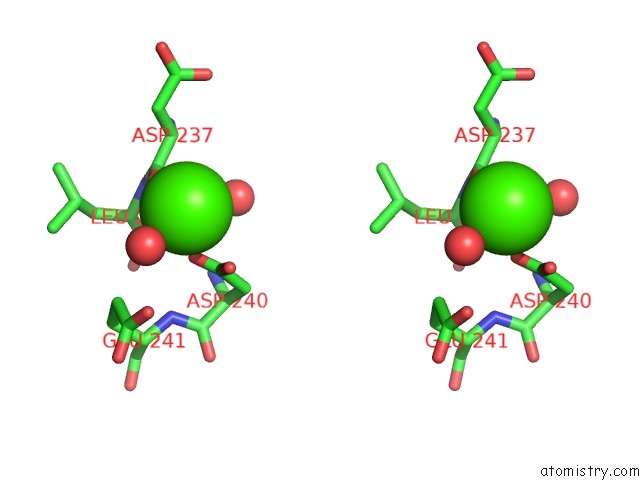
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of the E. Coli Phosphoglycerate Kinase within 5.0Å range:
|
Calcium binding site 2 out of 3 in 1zmr
Go back to
Calcium binding site 2 out
of 3 in the Crystal Structure of the E. Coli Phosphoglycerate Kinase
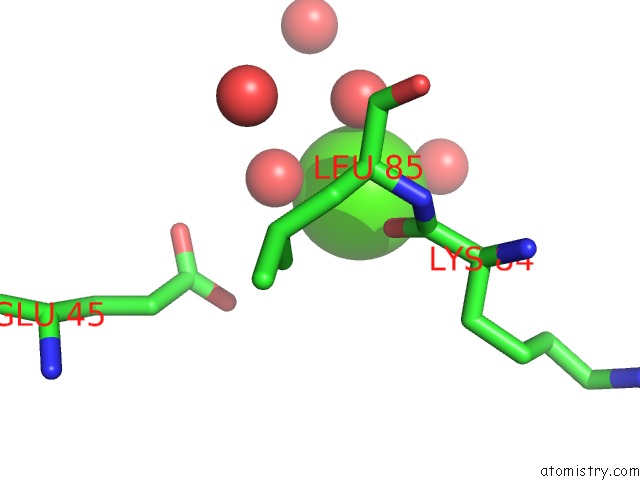
Mono view
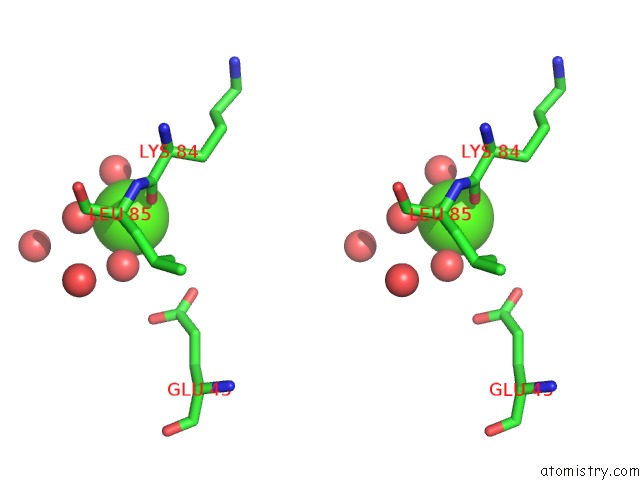
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Crystal Structure of the E. Coli Phosphoglycerate Kinase within 5.0Å range:
|
Calcium binding site 3 out of 3 in 1zmr
Go back to
Calcium binding site 3 out
of 3 in the Crystal Structure of the E. Coli Phosphoglycerate Kinase
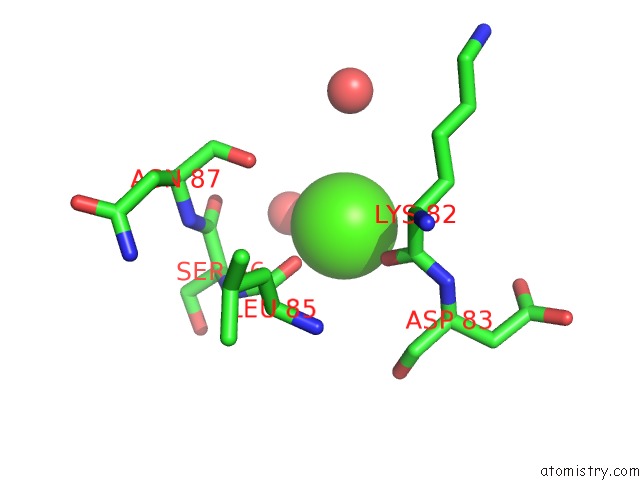
Mono view
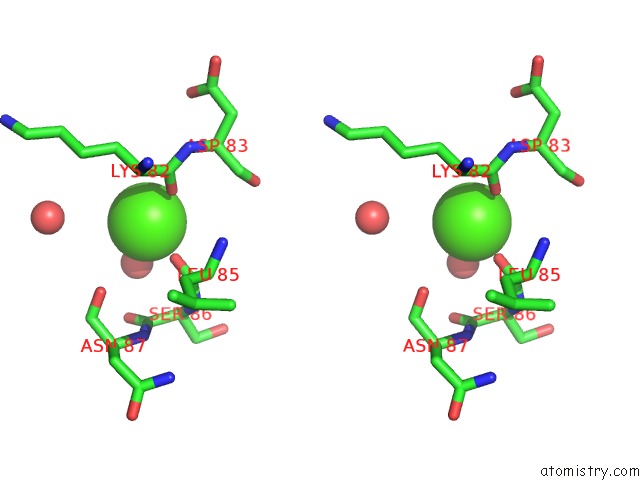
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Crystal Structure of the E. Coli Phosphoglycerate Kinase within 5.0Å range:
|
Reference:
T.A.Young,
E.Skordalakes,
S.Marqusee.
Comparison of Proteolytic Susceptibility in Phosphoglycerate Kinases From Yeast and E. Coli: Modulation of Conformational Ensembles Without Altering Structure or Stability. J.Mol.Biol. V. 368 1438 2007.
ISSN: ISSN 0022-2836
PubMed: 17397866
DOI: 10.1016/J.JMB.2007.02.077
Page generated: Tue Jul 8 04:05:40 2025
ISSN: ISSN 0022-2836
PubMed: 17397866
DOI: 10.1016/J.JMB.2007.02.077
Last articles
Cl in 5HLLCl in 5HLS
Cl in 5HKO
Cl in 5HKA
Cl in 5HLI
Cl in 5HL3
Cl in 5HK9
Cl in 5HKY
Cl in 5HKG
Cl in 5HK7