Calcium »
PDB 2vvc-2w3j »
2w22 »
Calcium in PDB 2w22: Activation Mechanism of Bacterial Thermoalkalophilic Lipases
Enzymatic activity of Activation Mechanism of Bacterial Thermoalkalophilic Lipases
All present enzymatic activity of Activation Mechanism of Bacterial Thermoalkalophilic Lipases:
3.1.1.3;
3.1.1.3;
Protein crystallography data
The structure of Activation Mechanism of Bacterial Thermoalkalophilic Lipases, PDB code: 2w22
was solved by
C.Carrasco-Lopez,
C.Godoy,
B.De Las Rivas,
G.Fernandez-Lorente,
J.M.Palomo,
J.M.Guisan,
R.Fernandez-Lafuente,
J.A.Hermoso,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.18 / 2.20 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 73.070, 128.080, 127.490, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.2 / 22.6 |
Other elements in 2w22:
The structure of Activation Mechanism of Bacterial Thermoalkalophilic Lipases also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Calcium Binding Sites:
The binding sites of Calcium atom in the Activation Mechanism of Bacterial Thermoalkalophilic Lipases
(pdb code 2w22). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total only one binding site of Calcium was determined in the Activation Mechanism of Bacterial Thermoalkalophilic Lipases, PDB code: 2w22:
In total only one binding site of Calcium was determined in the Activation Mechanism of Bacterial Thermoalkalophilic Lipases, PDB code: 2w22:
Calcium binding site 1 out of 1 in 2w22
Go back to
Calcium binding site 1 out
of 1 in the Activation Mechanism of Bacterial Thermoalkalophilic Lipases
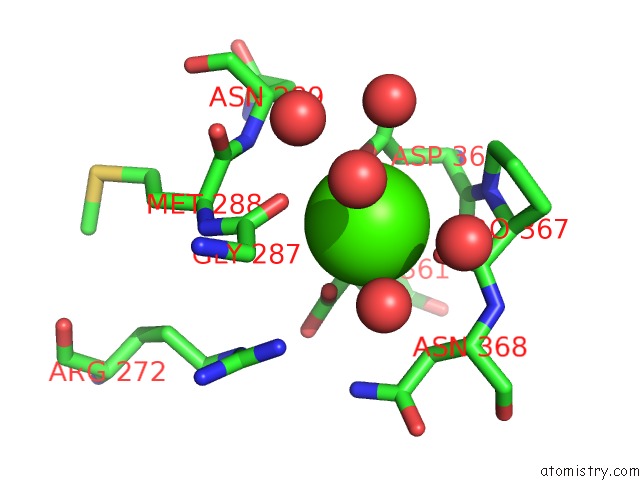
Mono view
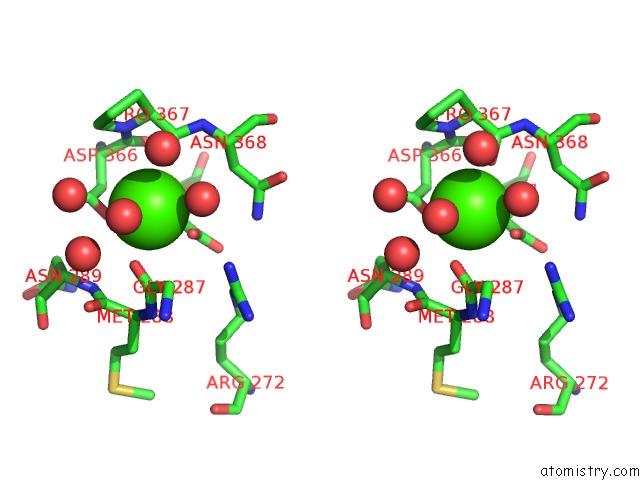
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Activation Mechanism of Bacterial Thermoalkalophilic Lipases within 5.0Å range:
|
Reference:
C.Carrasco-Lopez,
C.Godoy,
B.De Las Rivas,
G.Fernandez-Lorente,
J.M.Palomo,
J.M.Guisan,
R.Fernandez-Lafuente,
M.Martinez-Ripoll,
J.A.Hermoso.
Activation of Bacterial Thermoalkalophilic Lipases Is Spurred By Dramatic Structural Rearrangements. J. Biol. Chem. V. 284 4365 2009.
ISSN: ISSN 0021-9258
PubMed: 19056729
DOI: 10.1074/JBC.M808268200
Page generated: Tue Jul 8 08:54:24 2025
ISSN: ISSN 0021-9258
PubMed: 19056729
DOI: 10.1074/JBC.M808268200
Last articles
Cl in 8D7SCl in 8D7Q
Cl in 8D7L
Cl in 8D89
Cl in 8D88
Cl in 8D7D
Cl in 8D7J
Cl in 8D7C
Cl in 8D7B
Cl in 8D77