Calcium »
PDB 3gy6-3hii »
3h32 »
Calcium in PDB 3h32: Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide
Protein crystallography data
The structure of Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide, PDB code: 3h32
was solved by
R.F.Doolittle,
L.Pandi,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.00 / 3.60 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 264.720, 97.320, 132.490, 90.00, 122.78, 90.00 |
| R / Rfree (%) | 26.3 / 32 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide
(pdb code 3h32). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 4 binding sites of Calcium where determined in the Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide, PDB code: 3h32:
Jump to Calcium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Calcium where determined in the Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide, PDB code: 3h32:
Jump to Calcium binding site number: 1; 2; 3; 4;
Calcium binding site 1 out of 4 in 3h32
Go back to
Calcium binding site 1 out
of 4 in the Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide within 5.0Å range:
|
Calcium binding site 2 out of 4 in 3h32
Go back to
Calcium binding site 2 out
of 4 in the Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide
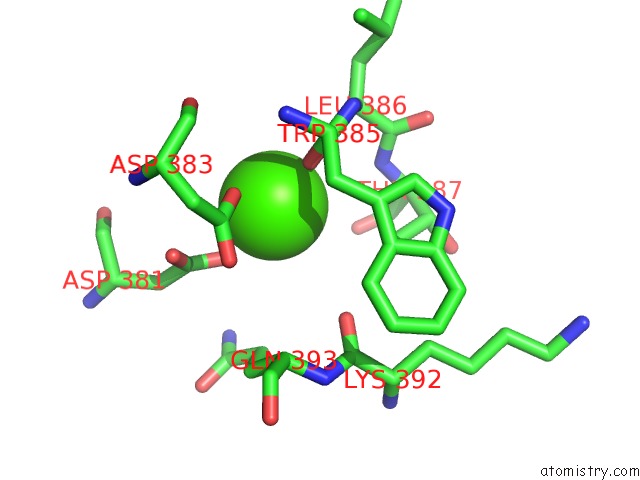
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide within 5.0Å range:
|
Calcium binding site 3 out of 4 in 3h32
Go back to
Calcium binding site 3 out
of 4 in the Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide

Mono view
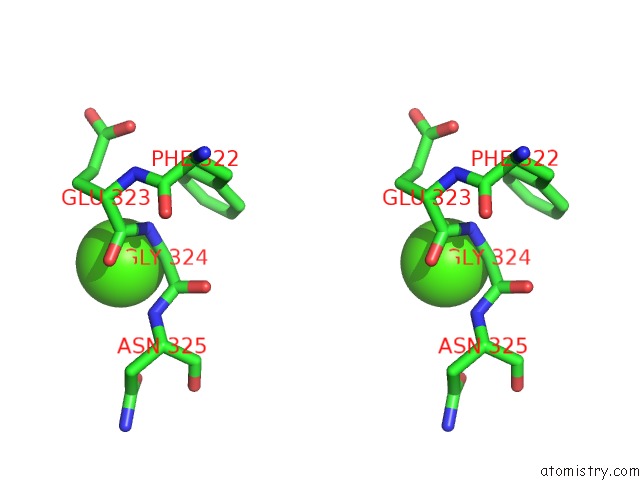
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide within 5.0Å range:
|
Calcium binding site 4 out of 4 in 3h32
Go back to
Calcium binding site 4 out
of 4 in the Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 4 of Crystal Structure of D-Dimer From Human Fibrin Complexed with Gly-His- Arg-Pro-Tyr-Amide within 5.0Å range:
|
Reference:
L.Pandi,
J.M.Kollman,
F.Lopez-Lira,
J.M.Burrows,
M.Riley,
R.F.Doolittle.
Two Families of Synthetic Peptides That Enhance Fibrin Turbidity and Delay Fibrinolysis By Different Mechanisms. Biochemistry V. 48 7201 2009.
ISSN: ISSN 0006-2960
PubMed: 19588915
DOI: 10.1021/BI900647G
Page generated: Tue Jul 8 12:51:30 2025
ISSN: ISSN 0006-2960
PubMed: 19588915
DOI: 10.1021/BI900647G
Last articles
Cl in 5K4ICl in 5K3C
Cl in 5K3B
Cl in 5K3E
Cl in 5K3F
Cl in 5K31
Cl in 5K3A
Cl in 5K0X
Cl in 5K0W
Cl in 5K2Z