Calcium »
PDB 3mis-3mz5 »
3msn »
Calcium in PDB 3msn: Crystal Structure of Thermolysin in Complex with N-Methylurea
Enzymatic activity of Crystal Structure of Thermolysin in Complex with N-Methylurea
All present enzymatic activity of Crystal Structure of Thermolysin in Complex with N-Methylurea:
3.4.24.27;
3.4.24.27;
Protein crystallography data
The structure of Crystal Structure of Thermolysin in Complex with N-Methylurea, PDB code: 3msn
was solved by
J.Behnen,
A.Heine,
G.Klebe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 10.00 / 1.97 |
| Space group | P 61 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 93.088, 93.088, 129.149, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 17.6 / 23.1 |
Other elements in 3msn:
The structure of Crystal Structure of Thermolysin in Complex with N-Methylurea also contains other interesting chemical elements:
| Zinc | (Zn) | 1 atom |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of Thermolysin in Complex with N-Methylurea
(pdb code 3msn). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 4 binding sites of Calcium where determined in the Crystal Structure of Thermolysin in Complex with N-Methylurea, PDB code: 3msn:
Jump to Calcium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Calcium where determined in the Crystal Structure of Thermolysin in Complex with N-Methylurea, PDB code: 3msn:
Jump to Calcium binding site number: 1; 2; 3; 4;
Calcium binding site 1 out of 4 in 3msn
Go back to
Calcium binding site 1 out
of 4 in the Crystal Structure of Thermolysin in Complex with N-Methylurea
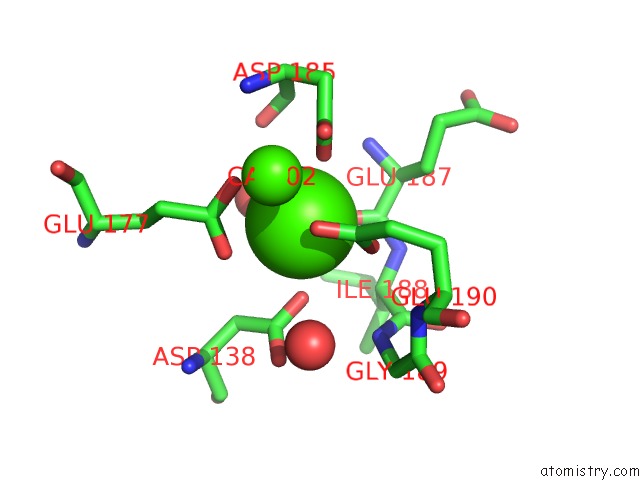
Mono view
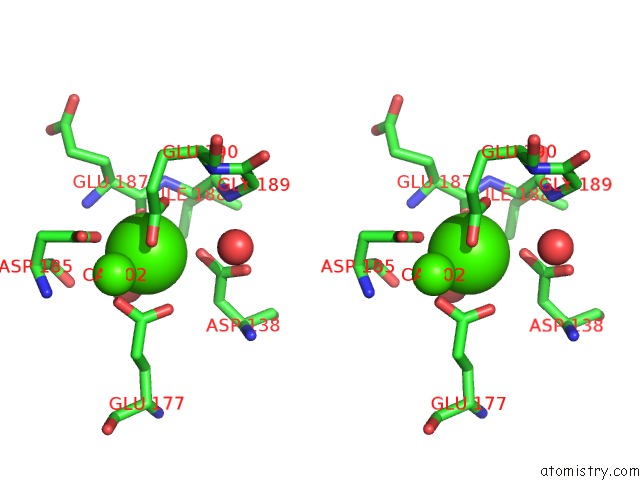
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of Thermolysin in Complex with N-Methylurea within 5.0Å range:
|
Calcium binding site 2 out of 4 in 3msn
Go back to
Calcium binding site 2 out
of 4 in the Crystal Structure of Thermolysin in Complex with N-Methylurea
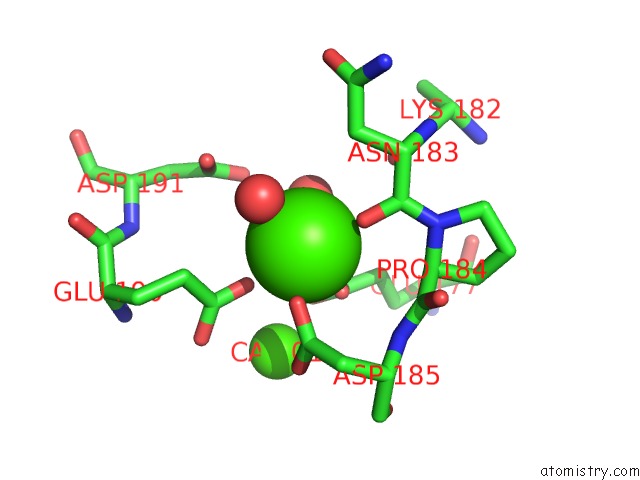
Mono view
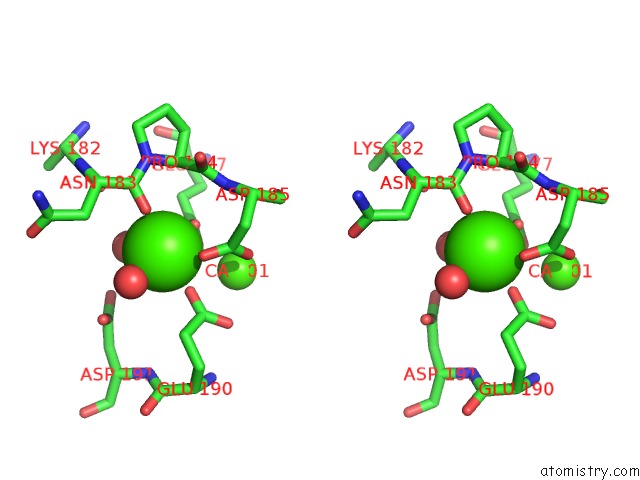
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Crystal Structure of Thermolysin in Complex with N-Methylurea within 5.0Å range:
|
Calcium binding site 3 out of 4 in 3msn
Go back to
Calcium binding site 3 out
of 4 in the Crystal Structure of Thermolysin in Complex with N-Methylurea
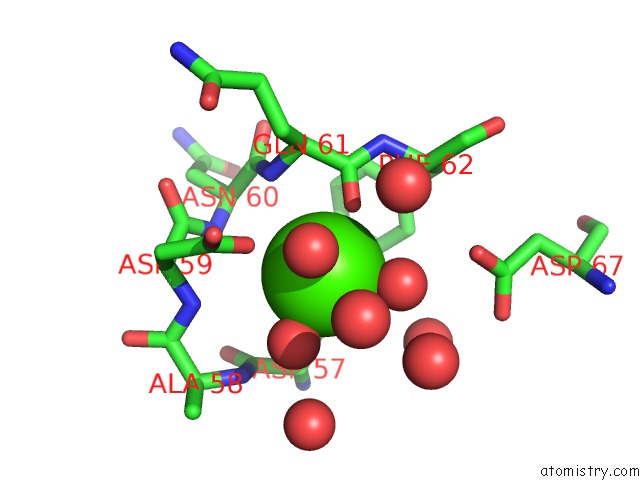
Mono view
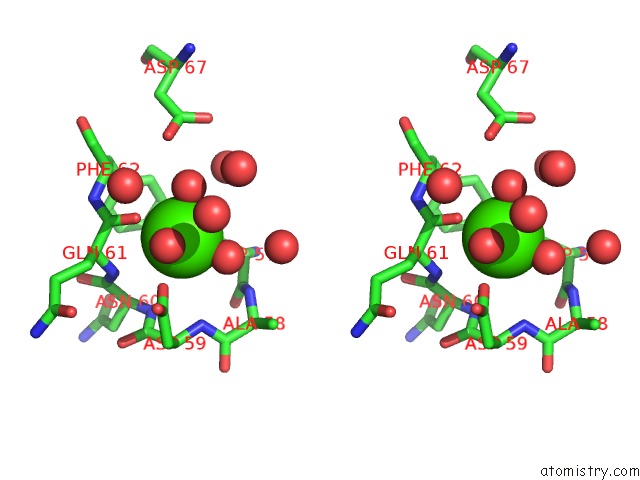
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Crystal Structure of Thermolysin in Complex with N-Methylurea within 5.0Å range:
|
Calcium binding site 4 out of 4 in 3msn
Go back to
Calcium binding site 4 out
of 4 in the Crystal Structure of Thermolysin in Complex with N-Methylurea
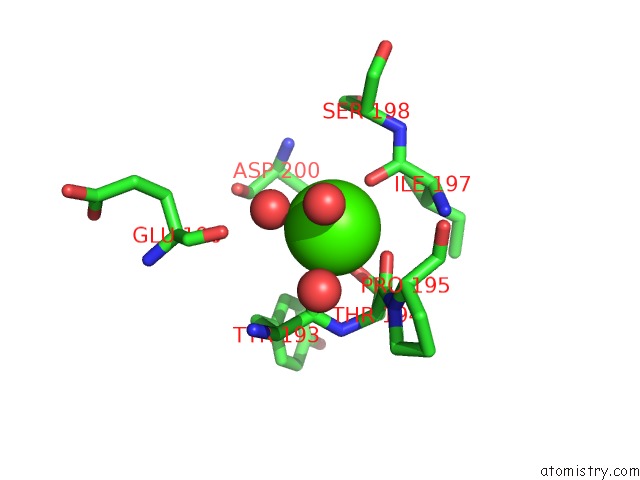
Mono view
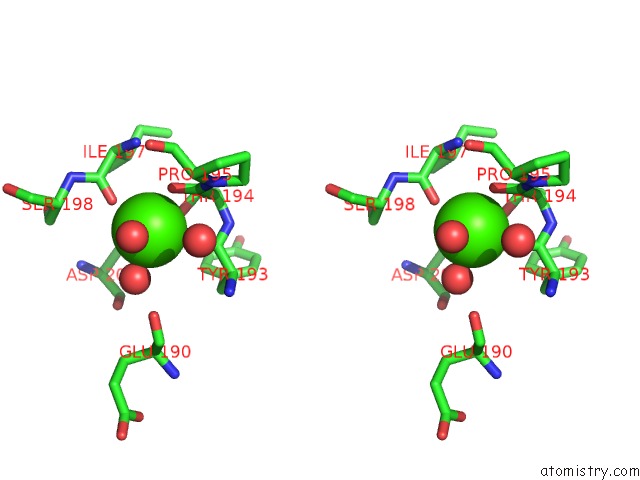
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 4 of Crystal Structure of Thermolysin in Complex with N-Methylurea within 5.0Å range:
|
Reference:
J.Behnen,
H.Koster,
G.Neudert,
T.Craan,
A.Heine,
G.Klebe.
Experimental and Computational Active Site Mapping As A Starting Point to Fragment-Based Lead Discovery. Chemmedchem V. 7 248 2012.
ISSN: ISSN 1860-7179
PubMed: 22213702
DOI: 10.1002/CMDC.201100490
Page generated: Tue Jul 8 14:40:55 2025
ISSN: ISSN 1860-7179
PubMed: 22213702
DOI: 10.1002/CMDC.201100490
Last articles
F in 7P4EF in 7P3C
F in 7P1R
F in 7P3J
F in 7P3G
F in 7P1E
F in 7OZX
F in 7P2S
F in 7OYH
F in 7OUH