Calcium »
PDB 3ngw-3nuc »
3njh »
Calcium in PDB 3njh: D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis.
Protein crystallography data
The structure of D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis., PDB code: 3njh
was solved by
J.Osipiuk,
R.Mulligan,
M.Bargassa,
F.Collart,
A.Joachimiak,
Midwest Centerfor Structural Genomics (Mcsg),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.30 / 1.94 |
| Space group | P 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 97.986, 97.986, 65.844, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 12.7 / 15.2 |
Other elements in 3njh:
The structure of D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis. also contains other interesting chemical elements:
| Sodium | (Na) | 2 atoms |
Calcium Binding Sites:
The binding sites of Calcium atom in the D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis.
(pdb code 3njh). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 4 binding sites of Calcium where determined in the D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis., PDB code: 3njh:
Jump to Calcium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Calcium where determined in the D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis., PDB code: 3njh:
Jump to Calcium binding site number: 1; 2; 3; 4;
Calcium binding site 1 out of 4 in 3njh
Go back to
Calcium binding site 1 out
of 4 in the D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis.
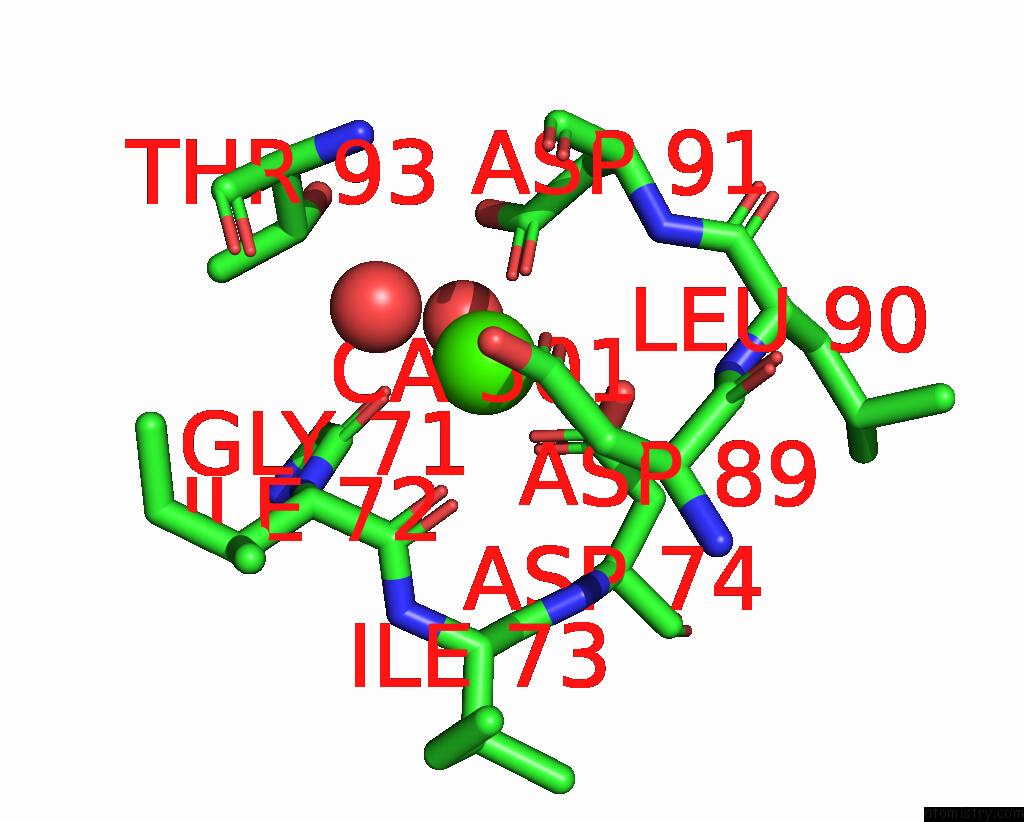
Mono view
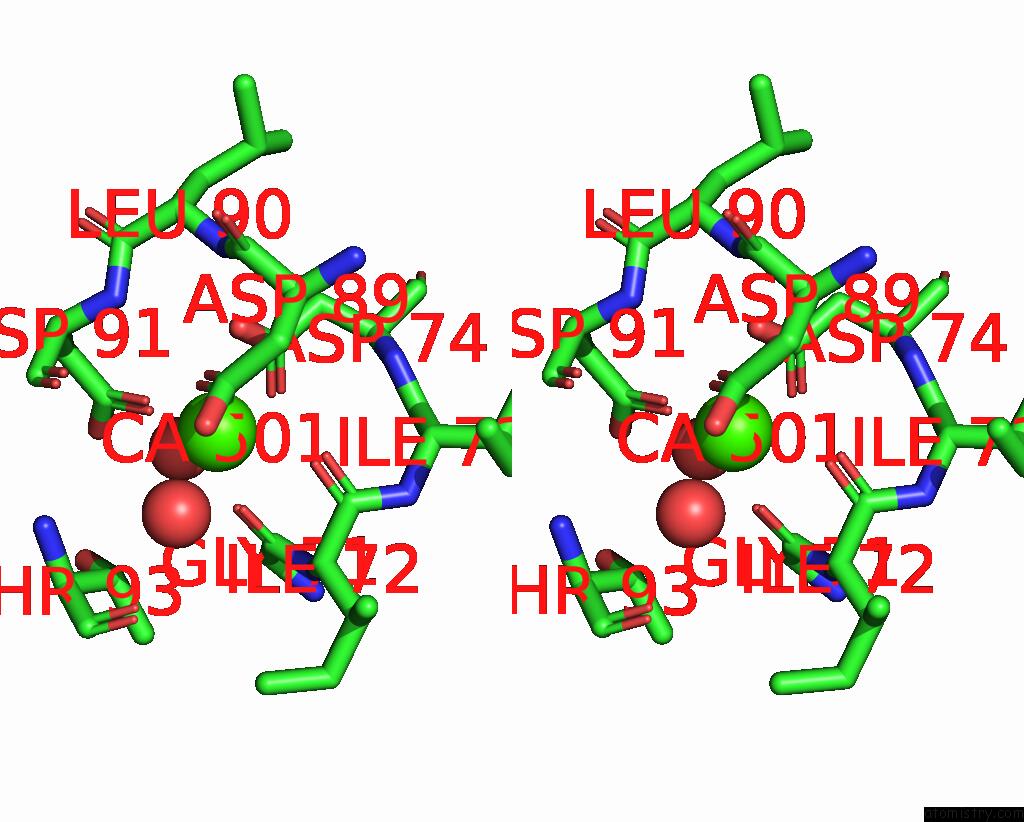
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis. within 5.0Å range:
|
Calcium binding site 2 out of 4 in 3njh
Go back to
Calcium binding site 2 out
of 4 in the D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis.
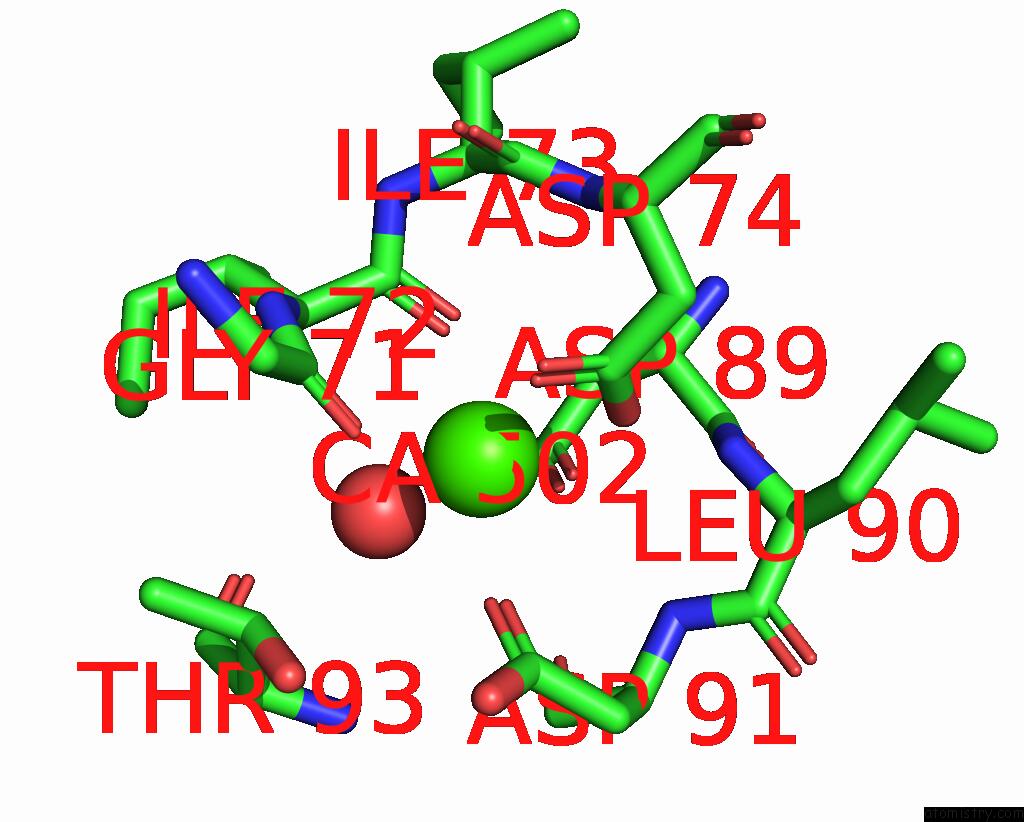
Mono view
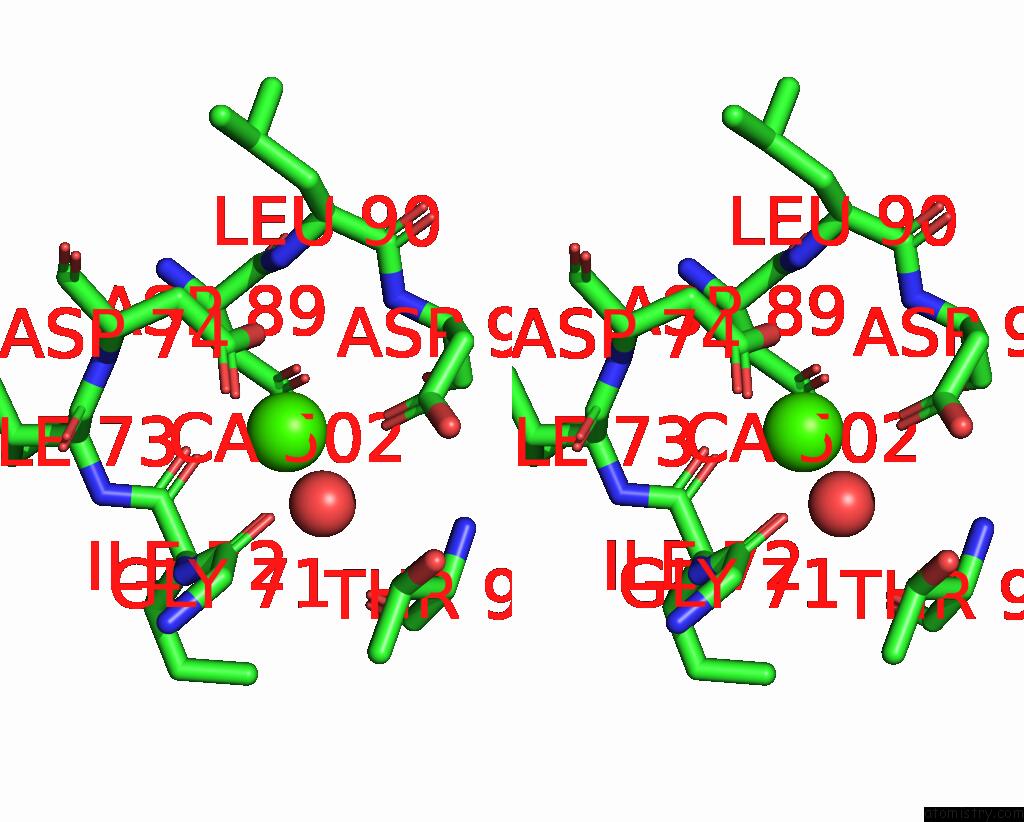
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis. within 5.0Å range:
|
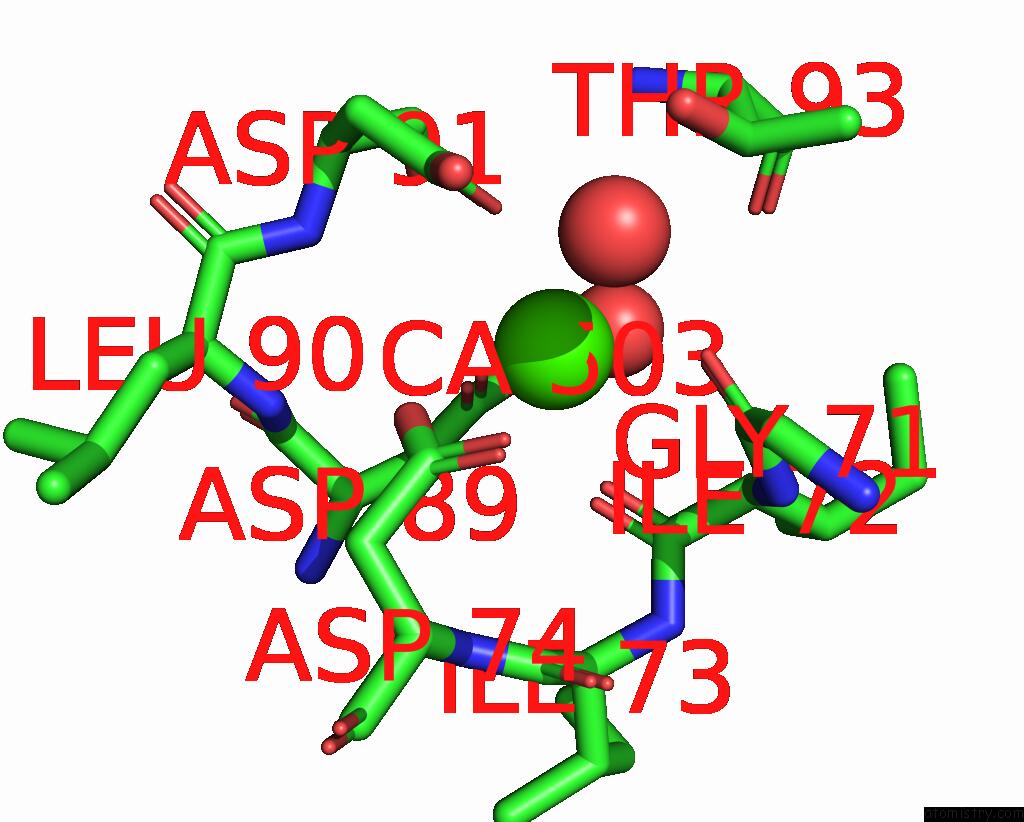
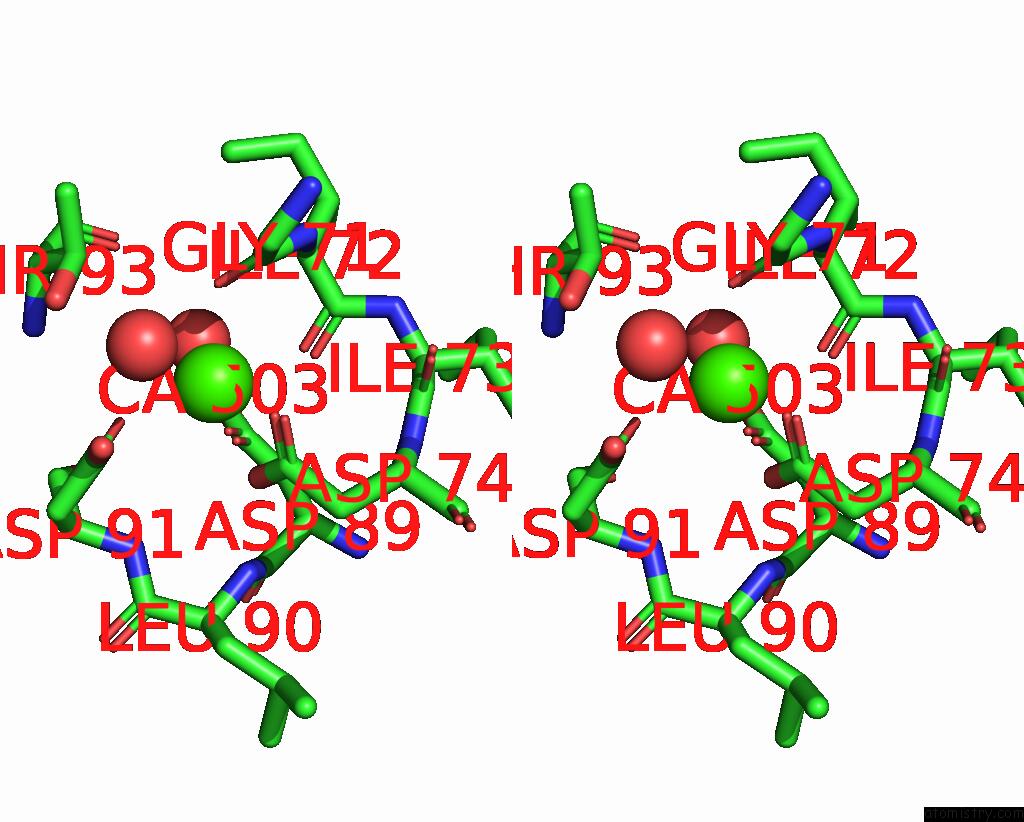
Calcium binding site 3 out of 4 in 3njh
Go back to
Calcium binding site 3 out
of 4 in the D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis.
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis. within 5.0Å range:
|
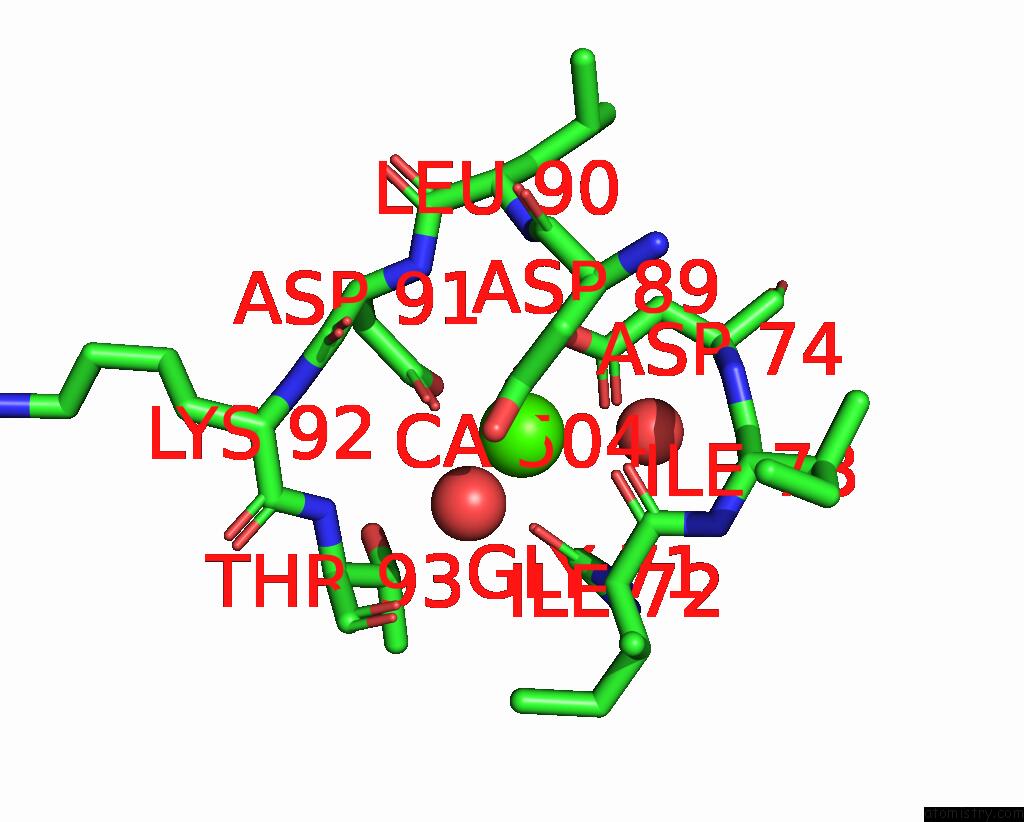
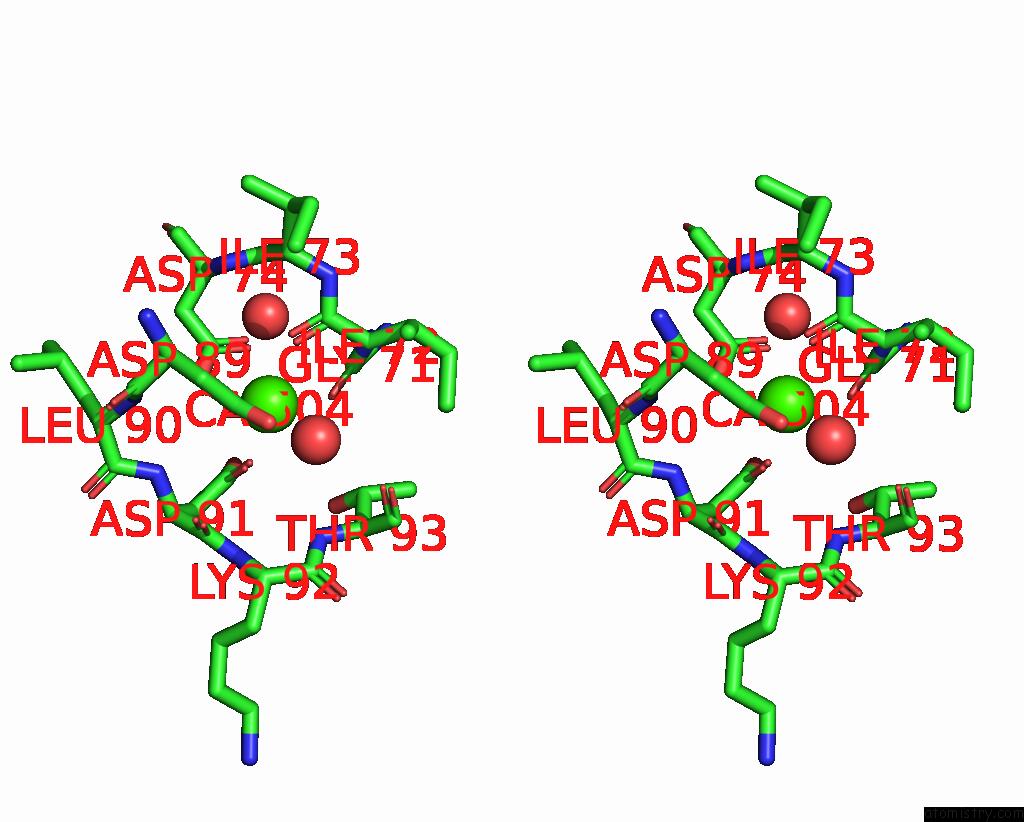
Calcium binding site 4 out of 4 in 3njh
Go back to
Calcium binding site 4 out
of 4 in the D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis.
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 4 of D37A Mutant of SO1698 Protein, An Aspartic Peptidase From Shewanella Oneidensis. within 5.0Å range:
|
Reference:
J.Osipiuk,
R.Mulligan,
M.Bargassa,
J.E.Hamilton,
M.A.Cunningham,
A.Joachimiak.
Characterization of Member of DUF1888 Protein Family, Self-Cleaving and Self-Assembling Endopeptidase. J.Biol.Chem. V. 287 19452 2012.
ISSN: ISSN 0021-9258
PubMed: 22493430
DOI: 10.1074/JBC.M112.358069
Page generated: Sat Jul 13 14:59:59 2024
ISSN: ISSN 0021-9258
PubMed: 22493430
DOI: 10.1074/JBC.M112.358069
Last articles
Zn in 9J0NZn in 9J0O
Zn in 9J0P
Zn in 9FJX
Zn in 9EKB
Zn in 9C0F
Zn in 9CAH
Zn in 9CH0
Zn in 9CH3
Zn in 9CH1