Calcium »
PDB 4y9p-4yty »
4yic »
Calcium in PDB 4yic: Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid
Protein crystallography data
The structure of Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid, PDB code: 4yic
was solved by
M.W.Vetting,
N.F.Al Obaidi,
R.Toro,
L.L.Morisco,
J.Benach,
J.Koss,
S.R.Wasserman,
J.D.Attonito,
A.Scott Glenn,
S.Chamala,
S.Chowdhury,
J.Lafleur,
J.Love,
R.D.Seidel,
K.L.Whalen,
J.A.Gerlt,
S.C.Almo,
Enzymefunction Initiative (Efi),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.56 / 1.60 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 64.681, 86.141, 72.339, 90.00, 100.86, 90.00 |
| R / Rfree (%) | 14.3 / 17.3 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid
(pdb code 4yic). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 2 binding sites of Calcium where determined in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid, PDB code: 4yic:
Jump to Calcium binding site number: 1; 2;
In total 2 binding sites of Calcium where determined in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid, PDB code: 4yic:
Jump to Calcium binding site number: 1; 2;
Calcium binding site 1 out of 2 in 4yic
Go back to
Calcium binding site 1 out
of 2 in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid
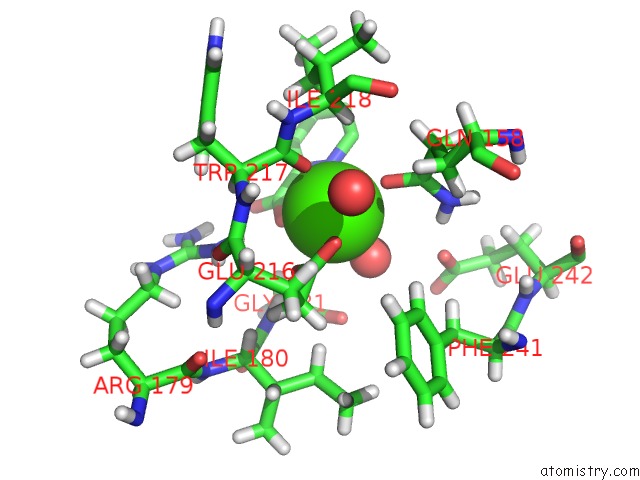
Mono view
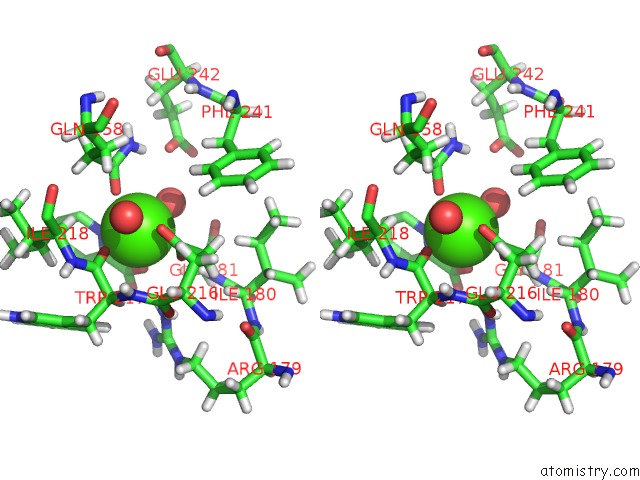
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid within 5.0Å range:
|
Calcium binding site 2 out of 2 in 4yic
Go back to
Calcium binding site 2 out
of 2 in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid
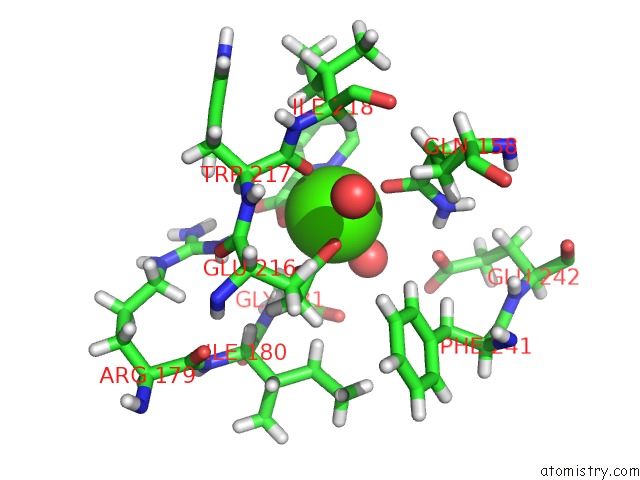
Mono view
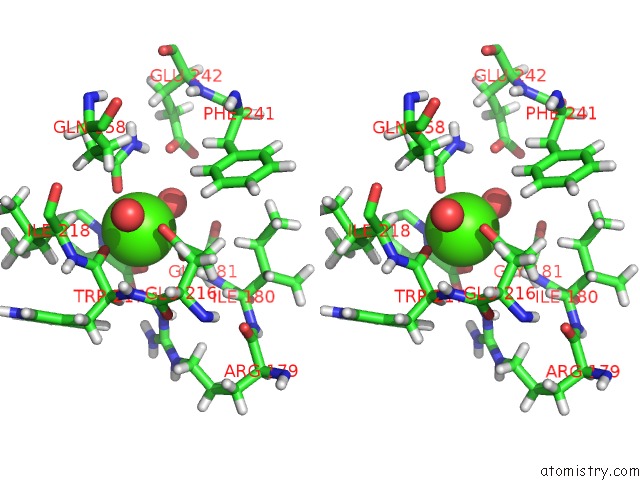
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid within 5.0Å range:
|
Reference:
M.W.Vetting,
N.F.Al Obaidi,
R.Toro,
L.L.Morisco,
J.Benach,
J.Koss,
S.R.Wasserman,
J.D.Attonito,
A.Scott Glenn,
S.Chamala,
S.Chowdhury,
J.Lafleur,
J.Love,
R.D.Seidel,
K.L.Whalen,
J.A.Gerlt,
S.C.Almo,
Enzyme Function Initiative (Efi).
Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi-500035) with Bound Picolinic Acid To Be Published.
Page generated: Wed Jul 9 03:12:05 2025
Last articles
Cl in 5I56Cl in 5I4Z
Cl in 5I53
Cl in 5I4X
Cl in 5I4Y
Cl in 5I4U
Cl in 5I4K
Cl in 5I4Q
Cl in 5I1T
Cl in 5I3W