Calcium »
PDB 5c02-5ck1 »
5cis »
Calcium in PDB 5cis: The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+
Protein crystallography data
The structure of The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+, PDB code: 5cis
was solved by
R.Nan,
C.M.Furze,
D.W.Wright,
J.Gor,
R.Wallis,
S.J.Perkins,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 50.34 / 2.58 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 66.930, 98.300, 121.410, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20 / 24.1 |
Calcium Binding Sites:
The binding sites of Calcium atom in the The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+
(pdb code 5cis). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 3 binding sites of Calcium where determined in the The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+, PDB code: 5cis:
Jump to Calcium binding site number: 1; 2; 3;
In total 3 binding sites of Calcium where determined in the The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+, PDB code: 5cis:
Jump to Calcium binding site number: 1; 2; 3;
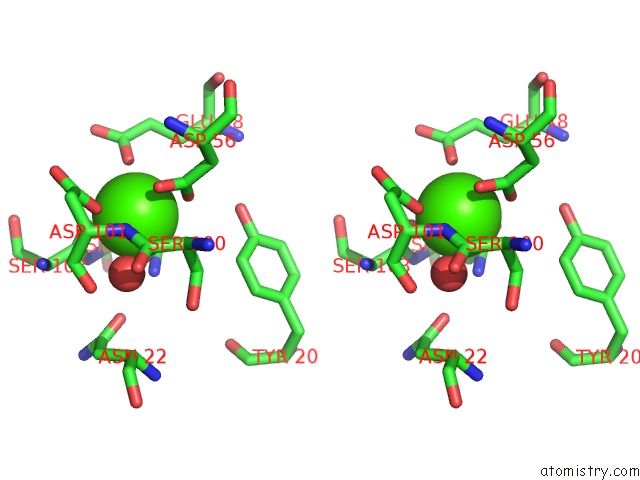
Calcium binding site 1 out of 3 in 5cis
Go back to
Calcium binding site 1 out
of 3 in the The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+ within 5.0Å range:
|
Calcium binding site 2 out of 3 in 5cis
Go back to
Calcium binding site 2 out
of 3 in the The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+ within 5.0Å range:
|
Calcium binding site 3 out of 3 in 5cis
Go back to
Calcium binding site 3 out
of 3 in the The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of The CUB1-Egf-CUB2 Domains of Rat Mbl-Associated Serine Protease-2 (Masp-2) Bound to CA2+ within 5.0Å range:
|
Reference:
R.Nan,
C.M.Furze,
D.W.Wright,
J.Gor,
R.Wallis,
S.J.Perkins.
Flexibility in Mannan-Binding Lectin-Associated Serine Proteases-1 and -2 Provides Insight on Lectin Pathway Activation. Structure V. 25 364 2017.
ISSN: ISSN 1878-4186
PubMed: 28111019
DOI: 10.1016/J.STR.2016.12.014
Page generated: Wed Jul 9 04:36:05 2025
ISSN: ISSN 1878-4186
PubMed: 28111019
DOI: 10.1016/J.STR.2016.12.014
Last articles
F in 4CMUF in 4CMG
F in 4CMB
F in 4CMT
F in 4CM4
F in 4CMO
F in 4CKR
F in 4CFN
F in 4CLJ
F in 4CLI