Calcium »
PDB 5mi5-5mop »
5mnq »
Calcium in PDB 5mnq: Cationic Trypsin in Complex with A Derivative of N-Amidinopiperidine
Enzymatic activity of Cationic Trypsin in Complex with A Derivative of N-Amidinopiperidine
All present enzymatic activity of Cationic Trypsin in Complex with A Derivative of N-Amidinopiperidine:
3.4.21.4;
3.4.21.4;
Protein crystallography data
The structure of Cationic Trypsin in Complex with A Derivative of N-Amidinopiperidine, PDB code: 5mnq
was solved by
J.Schiebel,
K.Ngo,
A.Heine,
G.Klebe,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.35 / 1.34 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 59.893, 64.076, 69.435, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 13.7 / 17.1 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Cationic Trypsin in Complex with A Derivative of N-Amidinopiperidine
(pdb code 5mnq). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total only one binding site of Calcium was determined in the Cationic Trypsin in Complex with A Derivative of N-Amidinopiperidine, PDB code: 5mnq:
In total only one binding site of Calcium was determined in the Cationic Trypsin in Complex with A Derivative of N-Amidinopiperidine, PDB code: 5mnq:
Calcium binding site 1 out of 1 in 5mnq
Go back to
Calcium binding site 1 out
of 1 in the Cationic Trypsin in Complex with A Derivative of N-Amidinopiperidine
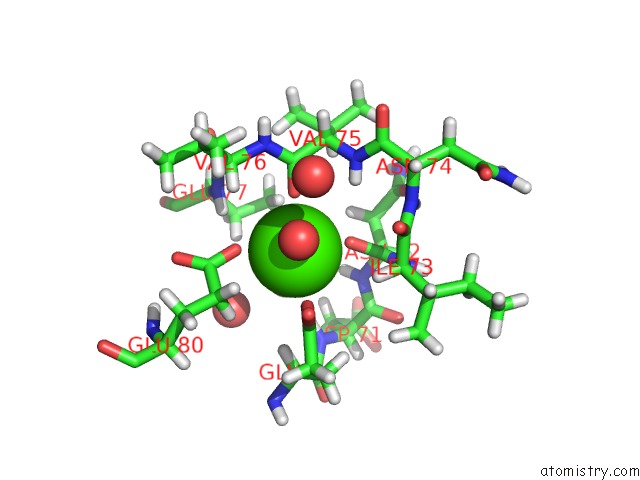
Mono view
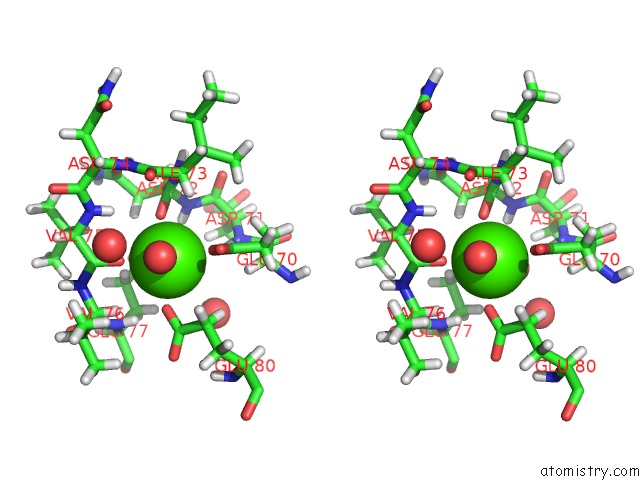
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Cationic Trypsin in Complex with A Derivative of N-Amidinopiperidine within 5.0Å range:
|
Reference:
J.Schiebel,
R.Gaspari,
T.Wulsdorf,
K.Ngo,
C.Sohn,
T.E.Schrader,
A.Cavalli,
A.Ostermann,
A.Heine,
G.Klebe.
Intriguing Role of Water in Protein-Ligand Binding Studied By Neutron Crystallography on Trypsin Complexes. Nat Commun V. 9 3559 2018.
ISSN: ESSN 2041-1723
PubMed: 30177695
DOI: 10.1038/S41467-018-05769-2
Page generated: Wed Jul 9 08:28:22 2025
ISSN: ESSN 2041-1723
PubMed: 30177695
DOI: 10.1038/S41467-018-05769-2
Last articles
Cl in 5IHRCl in 5IGN
Cl in 5IH5
Cl in 5IG6
Cl in 5IFU
Cl in 5IFT
Cl in 5IFP
Cl in 5IF8
Cl in 5IEZ
Cl in 5IFB