Calcium »
PDB 5xkf-5xu9 »
5xu9 »
Calcium in PDB 5xu9: Crystal Structure of Transketolase in Complex with Tpp Intermediate IX and Gauche Form Erythrose-4-Phosphate From Pichia Stipitis
Enzymatic activity of Crystal Structure of Transketolase in Complex with Tpp Intermediate IX and Gauche Form Erythrose-4-Phosphate From Pichia Stipitis
All present enzymatic activity of Crystal Structure of Transketolase in Complex with Tpp Intermediate IX and Gauche Form Erythrose-4-Phosphate From Pichia Stipitis:
2.2.1.1;
2.2.1.1;
Protein crystallography data
The structure of Crystal Structure of Transketolase in Complex with Tpp Intermediate IX and Gauche Form Erythrose-4-Phosphate From Pichia Stipitis, PDB code: 5xu9
was solved by
T.L.Li,
N.S.Hsu,
Y.L.Wang,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.61 / 1.17 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 101.259, 186.041, 98.667, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 14.6 / 16.6 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of Transketolase in Complex with Tpp Intermediate IX and Gauche Form Erythrose-4-Phosphate From Pichia Stipitis
(pdb code 5xu9). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total only one binding site of Calcium was determined in the Crystal Structure of Transketolase in Complex with Tpp Intermediate IX and Gauche Form Erythrose-4-Phosphate From Pichia Stipitis, PDB code: 5xu9:
In total only one binding site of Calcium was determined in the Crystal Structure of Transketolase in Complex with Tpp Intermediate IX and Gauche Form Erythrose-4-Phosphate From Pichia Stipitis, PDB code: 5xu9:
Calcium binding site 1 out of 1 in 5xu9
Go back to
Calcium binding site 1 out
of 1 in the Crystal Structure of Transketolase in Complex with Tpp Intermediate IX and Gauche Form Erythrose-4-Phosphate From Pichia Stipitis
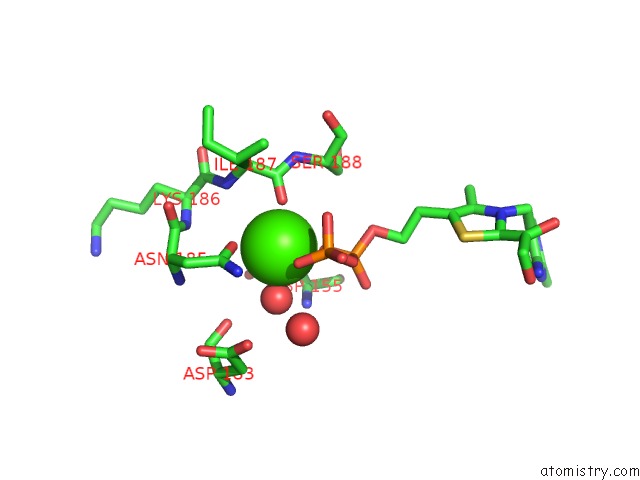
Mono view
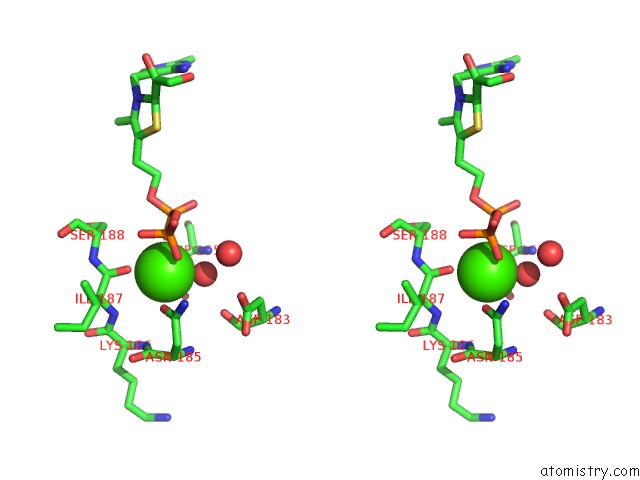
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of Transketolase in Complex with Tpp Intermediate IX and Gauche Form Erythrose-4-Phosphate From Pichia Stipitis within 5.0Å range:
|
Reference:
N.S.Hsu,
Y.L.Wang,
K.H.Lin,
C.F.Chang,
S.C.Ke,
S.Y.Lyu,
L.J.Hsu,
Y.S.Li,
S.C.Chen,
K.C.Wang,
T.L.Li.
Evidence of Diradicals Involved in the Yeast Transketolase Catalyzed Keto-Transferring Reactions. Chembiochem V. 19 2395 2018.
ISSN: ESSN 1439-7633
PubMed: 30155962
DOI: 10.1002/CBIC.201800378
Page generated: Wed Jul 9 11:47:44 2025
ISSN: ESSN 1439-7633
PubMed: 30155962
DOI: 10.1002/CBIC.201800378
Last articles
Ca in 7L74Ca in 7L2C
Ca in 7L6G
Ca in 7KXR
Ca in 7L68
Ca in 7L67
Ca in 7L66
Ca in 7L64
Ca in 7L65
Ca in 7L63