Calcium »
PDB 5zyv-6ahg »
5zz0 »
Calcium in PDB 5zz0: Human Gelsolin From Residues GLU28 to ARG161 with Calcium
Protein crystallography data
The structure of Human Gelsolin From Residues GLU28 to ARG161 with Calcium, PDB code: 5zz0
was solved by
P.Sharma,
M.Badmalia,
S.P.S.Yadav,
S.Singh,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.84 / 2.64 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 35.393, 63.566, 98.136, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.6 / 25.4 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Human Gelsolin From Residues GLU28 to ARG161 with Calcium
(pdb code 5zz0). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 4 binding sites of Calcium where determined in the Human Gelsolin From Residues GLU28 to ARG161 with Calcium, PDB code: 5zz0:
Jump to Calcium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Calcium where determined in the Human Gelsolin From Residues GLU28 to ARG161 with Calcium, PDB code: 5zz0:
Jump to Calcium binding site number: 1; 2; 3; 4;
Calcium binding site 1 out of 4 in 5zz0
Go back to
Calcium binding site 1 out
of 4 in the Human Gelsolin From Residues GLU28 to ARG161 with Calcium
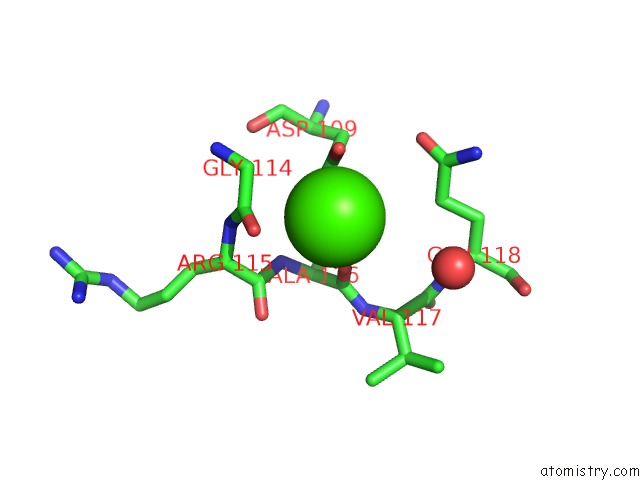
Mono view
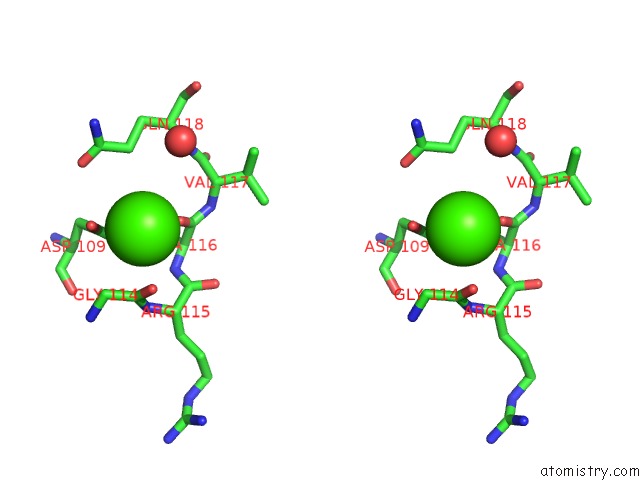
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Human Gelsolin From Residues GLU28 to ARG161 with Calcium within 5.0Å range:
|
Calcium binding site 2 out of 4 in 5zz0
Go back to
Calcium binding site 2 out
of 4 in the Human Gelsolin From Residues GLU28 to ARG161 with Calcium
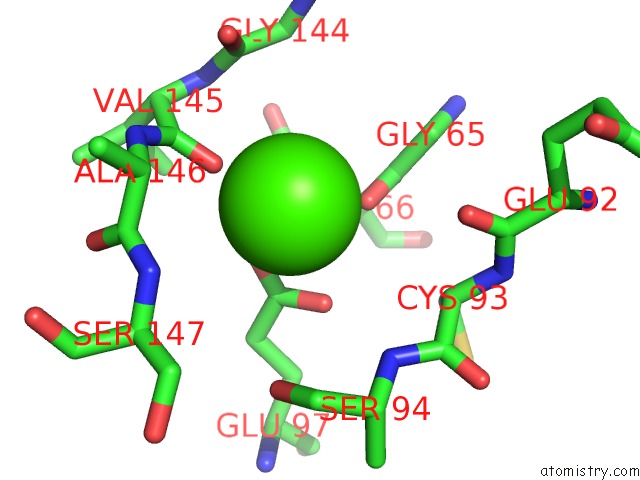
Mono view
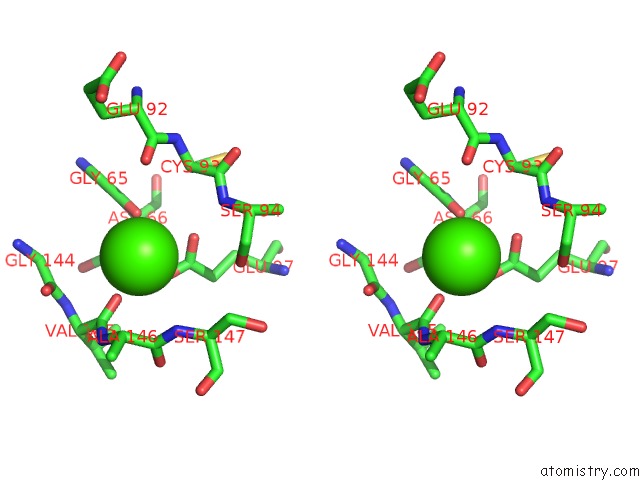
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Human Gelsolin From Residues GLU28 to ARG161 with Calcium within 5.0Å range:
|
Calcium binding site 3 out of 4 in 5zz0
Go back to
Calcium binding site 3 out
of 4 in the Human Gelsolin From Residues GLU28 to ARG161 with Calcium
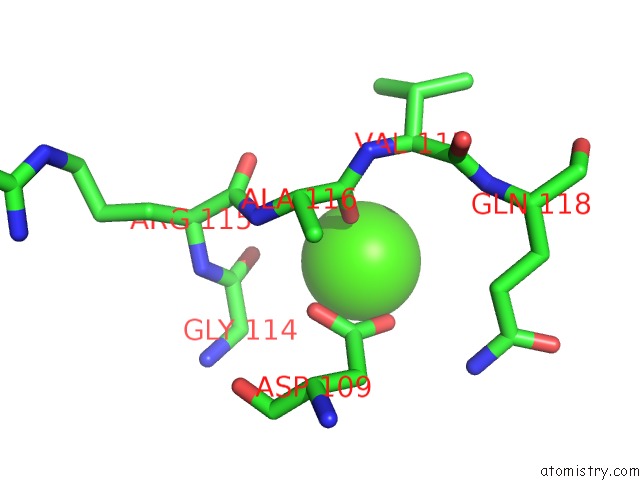
Mono view
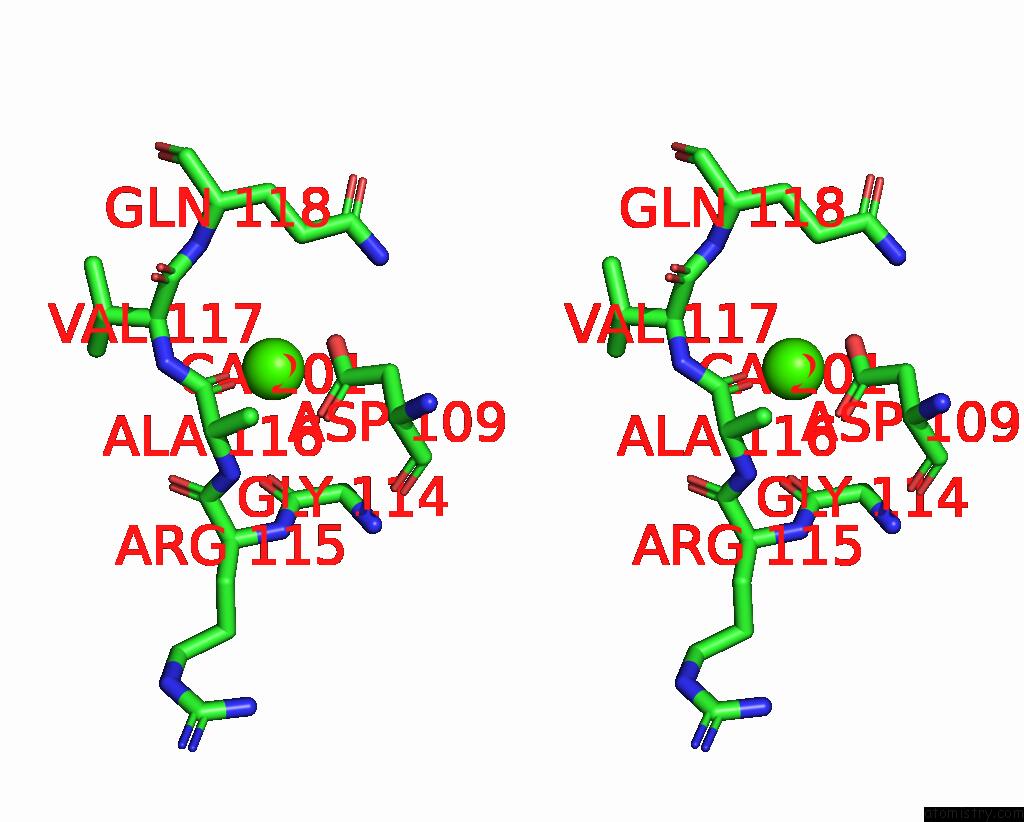
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Human Gelsolin From Residues GLU28 to ARG161 with Calcium within 5.0Å range:
|
Calcium binding site 4 out of 4 in 5zz0
Go back to
Calcium binding site 4 out
of 4 in the Human Gelsolin From Residues GLU28 to ARG161 with Calcium
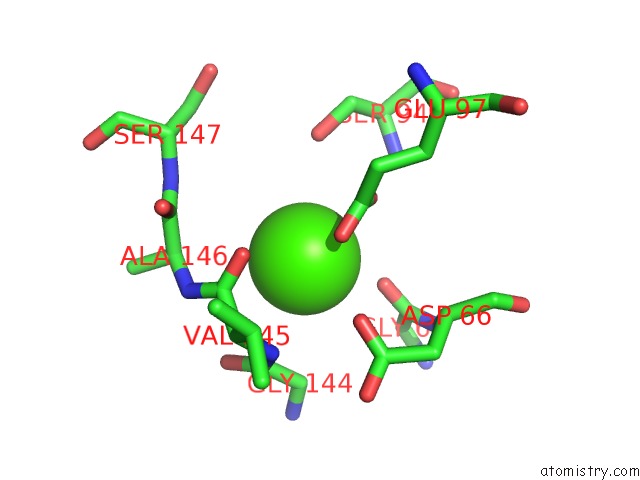
Mono view
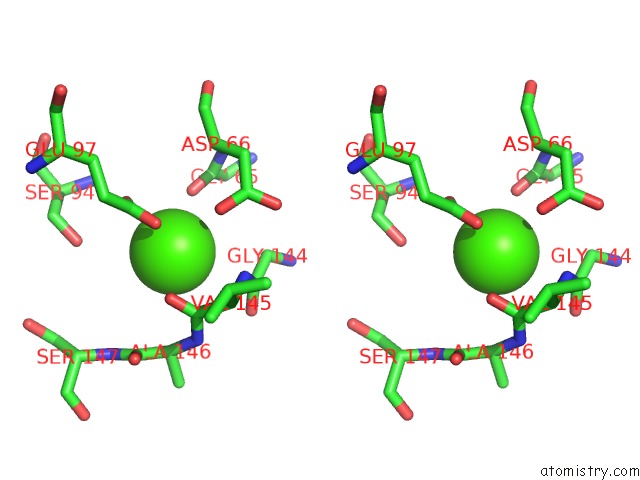
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 4 of Human Gelsolin From Residues GLU28 to ARG161 with Calcium within 5.0Å range:
|
Reference:
M.D.Badmalia,
P.Sharma,
S.P.S.Yadav,
S.Singh,
N.Khatri,
R.Garg.
Bonsai Gelsolin Survives Heat Induced Denaturation By Forming Beta-Amyloids Which Leach Out Functional Monomer. Sci Rep V. 8 12602 2018.
ISSN: ESSN 2045-2322
PubMed: 30135452
DOI: 10.1038/S41598-018-30951-3
Page generated: Wed Jul 9 12:18:51 2025
ISSN: ESSN 2045-2322
PubMed: 30135452
DOI: 10.1038/S41598-018-30951-3
Last articles
F in 4E7PF in 4EAD
F in 4E3N
F in 4E4X
F in 4E91
F in 4E5D
F in 4E1V
F in 4DLE
F in 4E28
F in 4DZ9