Calcium »
PDB 6ai0-6b40 »
6aw9 »
Calcium in PDB 6aw9: 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili
Protein crystallography data
The structure of 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili, PDB code: 6aw9
was solved by
S.Lovell,
N.Mehzabeen,
K.P.Battaile,
Y.Deng,
R.P.Hanzlik,
I.Shams,
J.Moskovitz,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 44.58 / 2.55 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 63.297, 102.410, 125.588, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.8 / 25.9 |
Calcium Binding Sites:
The binding sites of Calcium atom in the 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili
(pdb code 6aw9). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 3 binding sites of Calcium where determined in the 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili, PDB code: 6aw9:
Jump to Calcium binding site number: 1; 2; 3;
In total 3 binding sites of Calcium where determined in the 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili, PDB code: 6aw9:
Jump to Calcium binding site number: 1; 2; 3;
Calcium binding site 1 out of 3 in 6aw9
Go back to
Calcium binding site 1 out
of 3 in the 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili
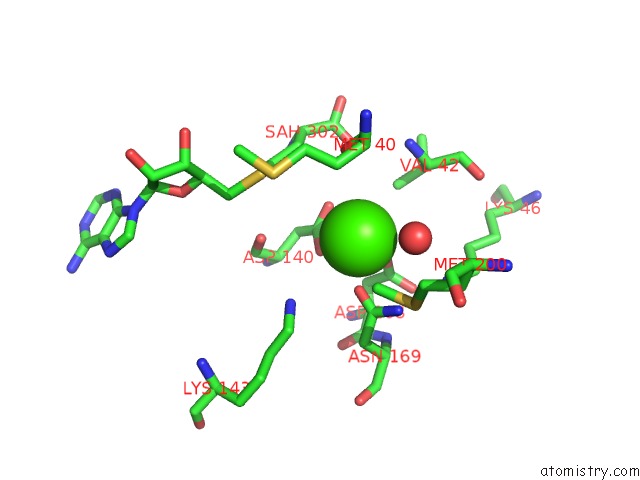
Mono view
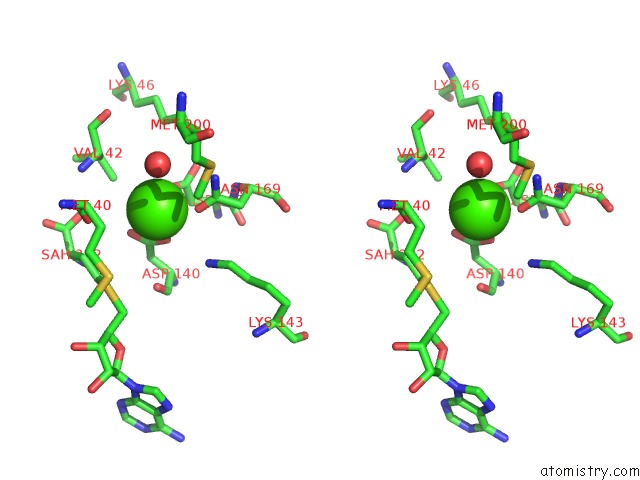
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili within 5.0Å range:
|
Calcium binding site 2 out of 3 in 6aw9
Go back to
Calcium binding site 2 out
of 3 in the 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili
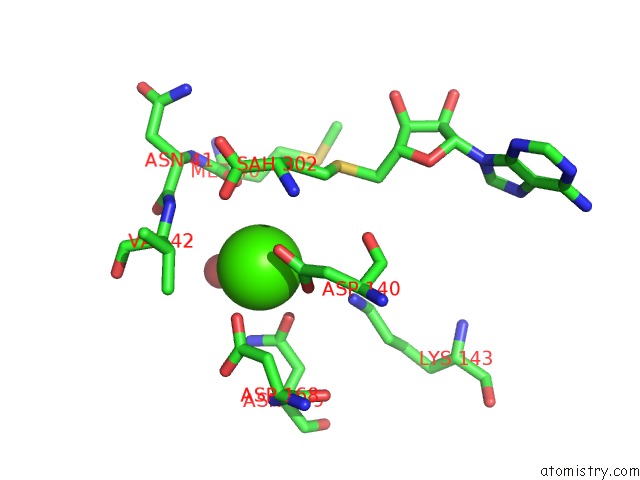
Mono view
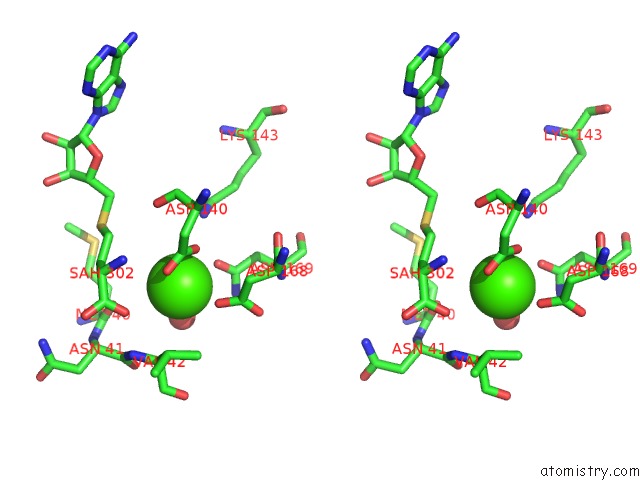
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili within 5.0Å range:
|
Calcium binding site 3 out of 3 in 6aw9
Go back to
Calcium binding site 3 out
of 3 in the 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili
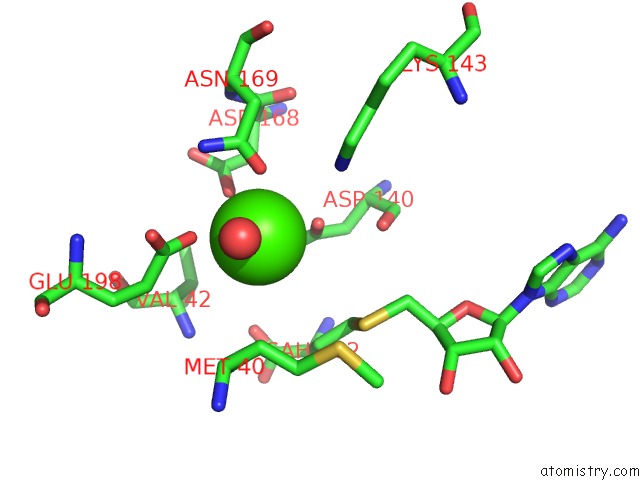
Mono view
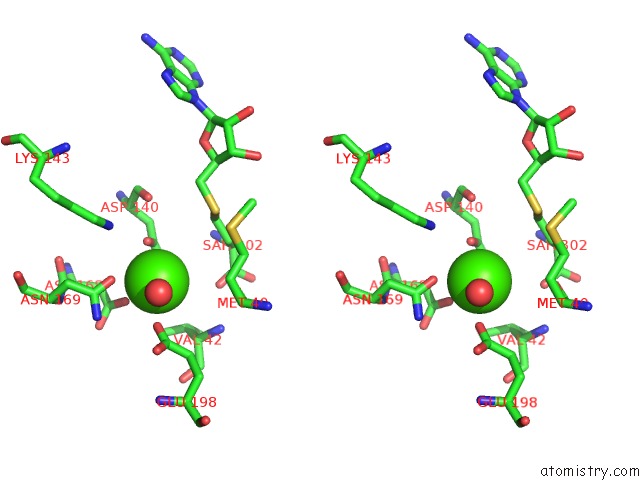
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of 2.55A Resolution Structure of Sah Bound Catechol O-Methyltransferase (Comt) L136M From Nannospalax Galili within 5.0Å range:
|
Reference:
Y.Deng,
S.Lovell,
N.Mehzabeen,
K.P.Battaile,
R.P.Hanzlik,
I.Shams,
J.Moskovitz.
Crystal Structure of the Catechol-O-Methyl Transferase (Comt) Enzyme of the Subterranean Mole Rat (Spalax) and the Effect of L136M Substitution To Be Published.
Page generated: Wed Jul 9 12:34:35 2025
Last articles
Cl in 8FBQCl in 8FB2
Cl in 8FBX
Cl in 8FB1
Cl in 8FAJ
Cl in 8FAV
Cl in 8FA0
Cl in 8FAX
Cl in 8F4Y
Cl in 8F8E