Calcium »
PDB 6fzw-6guy »
6fzw »
Calcium in PDB 6fzw: Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long)
Protein crystallography data
The structure of Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long), PDB code: 6fzw
was solved by
E.Hohenester,
D.Pulido,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 68.79 / 2.78 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 88.880, 143.650, 156.710, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 24.7 / 27.1 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long)
(pdb code 6fzw). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 5 binding sites of Calcium where determined in the Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long), PDB code: 6fzw:
Jump to Calcium binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Calcium where determined in the Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long), PDB code: 6fzw:
Jump to Calcium binding site number: 1; 2; 3; 4; 5;
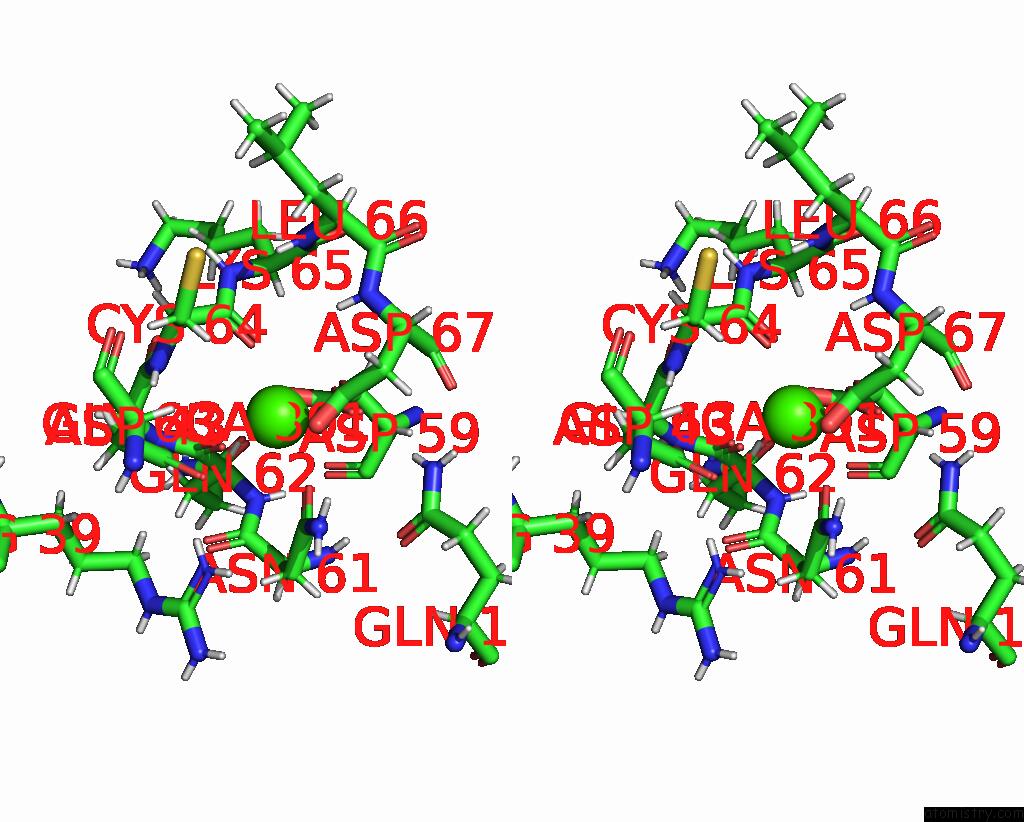
Calcium binding site 1 out of 5 in 6fzw
Go back to
Calcium binding site 1 out
of 5 in the Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long)

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long) within 5.0Å range:
|
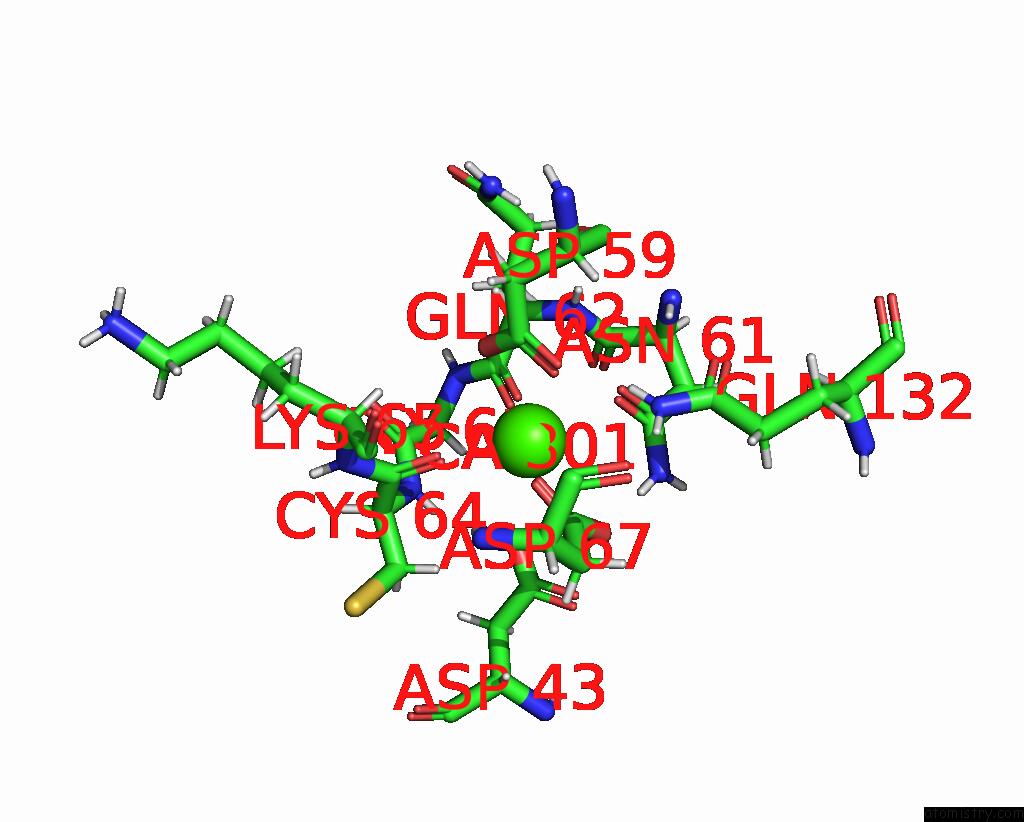
Calcium binding site 2 out of 5 in 6fzw
Go back to
Calcium binding site 2 out
of 5 in the Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long)
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long) within 5.0Å range:
|
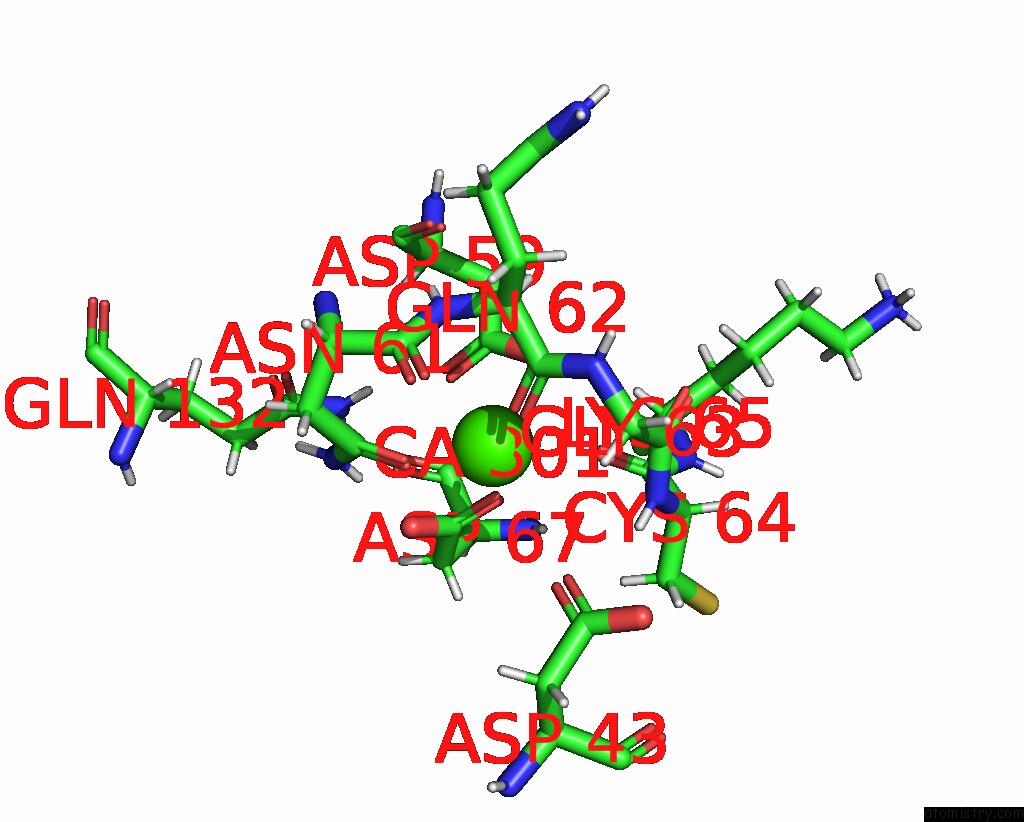
Calcium binding site 3 out of 5 in 6fzw
Go back to
Calcium binding site 3 out
of 5 in the Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long)
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long) within 5.0Å range:
|
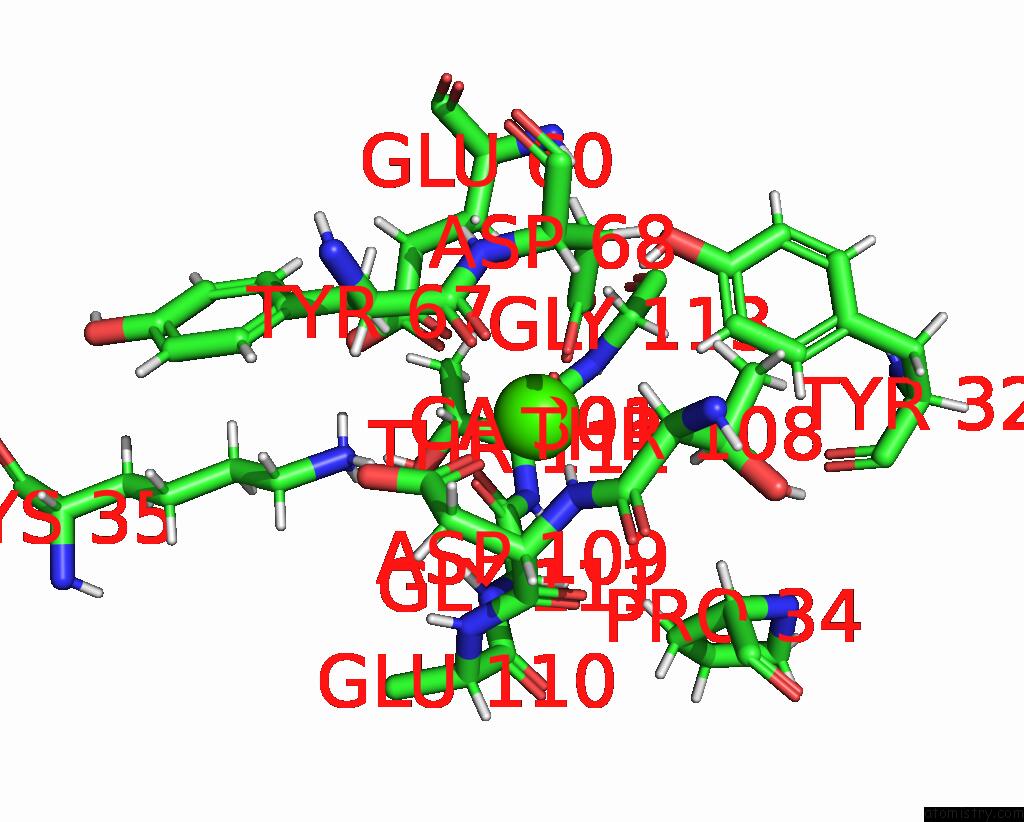
Calcium binding site 4 out of 5 in 6fzw
Go back to
Calcium binding site 4 out
of 5 in the Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long)
Mono view
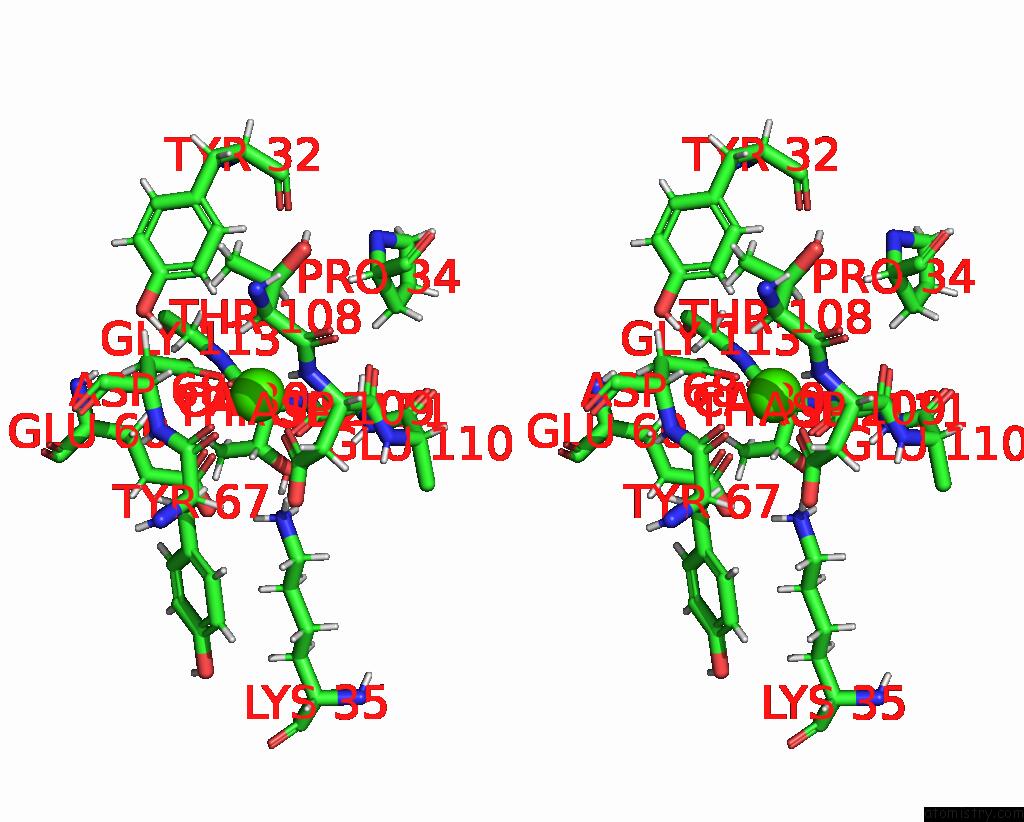
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 4 of Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long) within 5.0Å range:
|
Calcium binding site 5 out of 5 in 6fzw
Go back to
Calcium binding site 5 out
of 5 in the Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long)
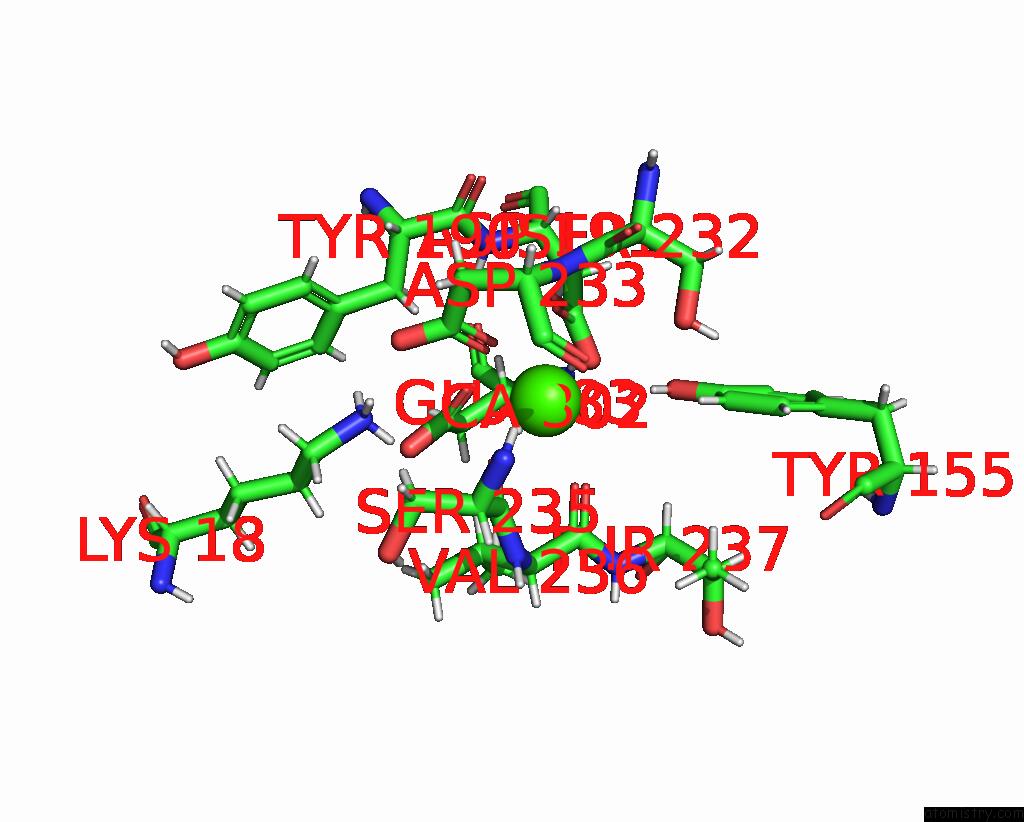
Mono view
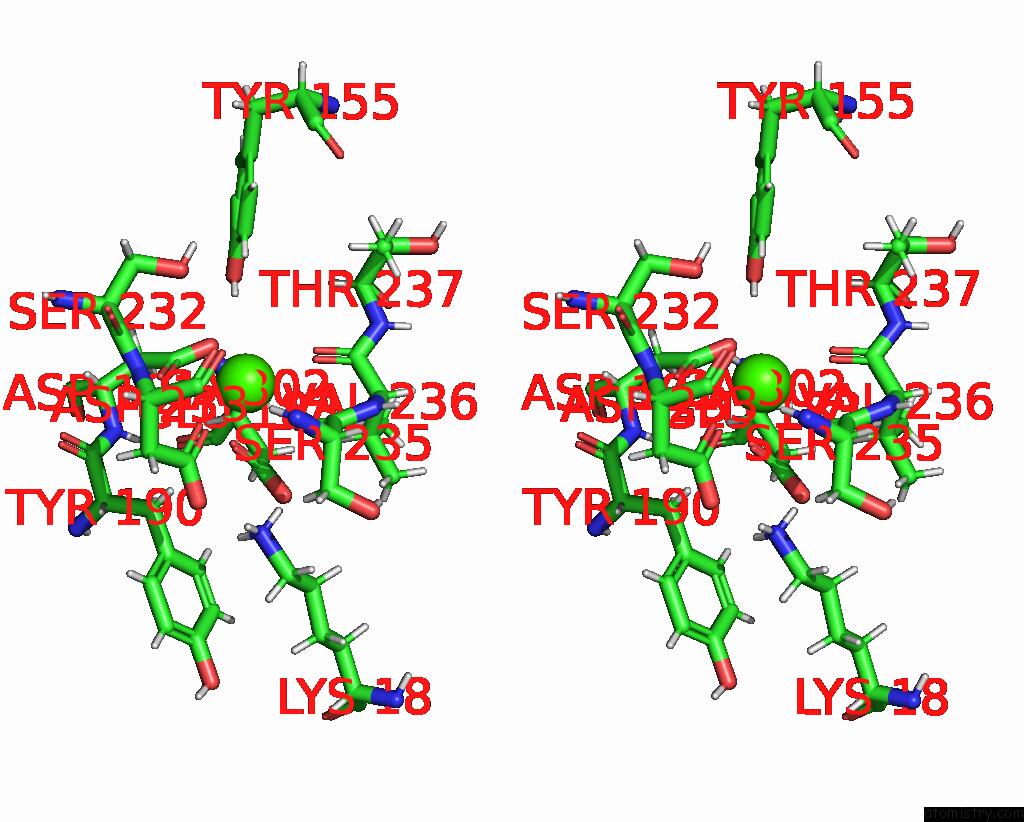
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 5 of Crystal Structure of the Metalloproteinase Enhancer Pcpe-1 Bound to the Procollagen C Propeptide Trimer (Long) within 5.0Å range:
|
Reference:
D.Pulido,
U.Sharma,
S.Vadon-Le Goff,
S.A.Hussain,
S.Cordes,
N.Mariano,
E.Bettler,
C.Moali,
N.Aghajari,
E.Hohenester,
D.J.S.Hulmes.
Structural Basis For the Acceleration of Procollagen Processing By Procollagen C-Proteinase Enhancer-1. Structure V. 26 1384 2018.
ISSN: ISSN 1878-4186
PubMed: 30078642
DOI: 10.1016/J.STR.2018.06.011
Page generated: Wed Jul 9 14:20:27 2025
ISSN: ISSN 1878-4186
PubMed: 30078642
DOI: 10.1016/J.STR.2018.06.011
Last articles
Cl in 8F58Cl in 8F55
Cl in 8F4Z
Cl in 8F57
Cl in 8F4K
Cl in 8F4T
Cl in 8F4U
Cl in 8F4S
Cl in 8F4J
Cl in 8F4Q