Calcium »
PDB 6guz-6hhz »
6h6v »
Calcium in PDB 6h6v: Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn
Protein crystallography data
The structure of Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn, PDB code: 6h6v
was solved by
K.A.P.Payne,
D.Leys,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 74.20 / 2.66 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 84.910, 139.250, 136.880, 90.00, 93.74, 90.00 |
| R / Rfree (%) | 19.9 / 23.6 |
Other elements in 6h6v:
The structure of Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn also contains other interesting chemical elements:
| Potassium | (K) | 6 atoms |
| Manganese | (Mn) | 6 atoms |
Calcium Binding Sites:
The binding sites of Calcium atom in the Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn
(pdb code 6h6v). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 6 binding sites of Calcium where determined in the Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn, PDB code: 6h6v:
Jump to Calcium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Calcium where determined in the Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn, PDB code: 6h6v:
Jump to Calcium binding site number: 1; 2; 3; 4; 5; 6;
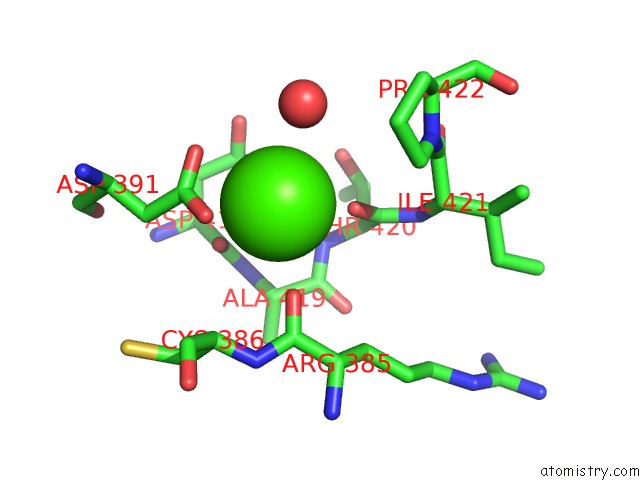
Calcium binding site 1 out of 6 in 6h6v
Go back to
Calcium binding site 1 out
of 6 in the Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn within 5.0Å range:
|
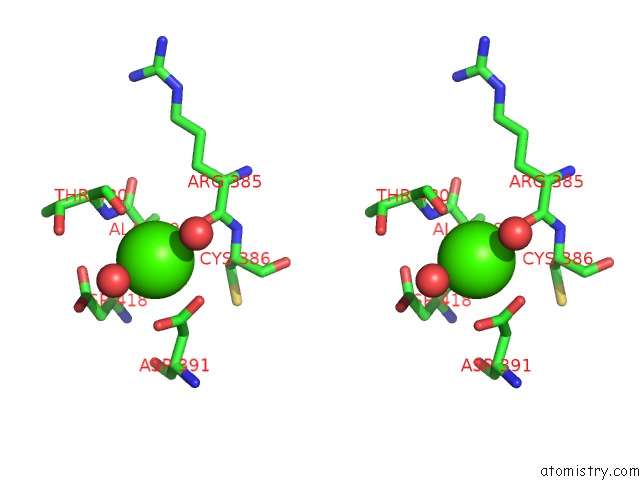
Calcium binding site 2 out of 6 in 6h6v
Go back to
Calcium binding site 2 out
of 6 in the Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn within 5.0Å range:
|
Calcium binding site 3 out of 6 in 6h6v
Go back to
Calcium binding site 3 out
of 6 in the Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn within 5.0Å range:
|
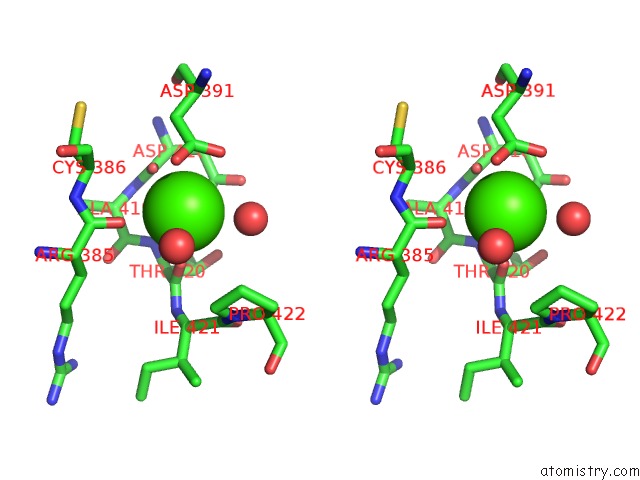
Calcium binding site 4 out of 6 in 6h6v
Go back to
Calcium binding site 4 out
of 6 in the Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 4 of Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn within 5.0Å range:
|
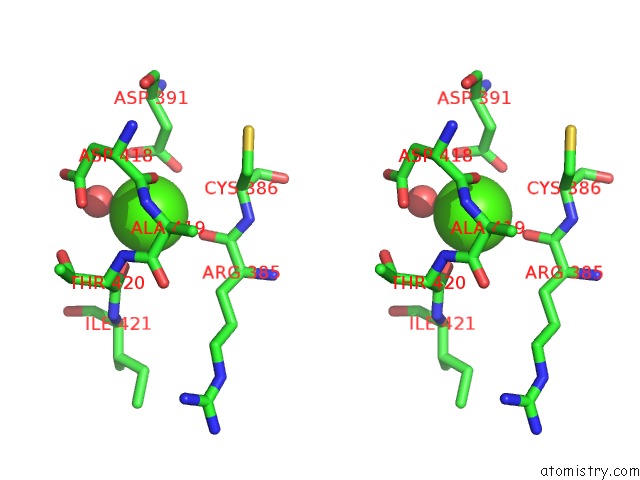
Calcium binding site 5 out of 6 in 6h6v
Go back to
Calcium binding site 5 out
of 6 in the Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn

Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 5 of Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn within 5.0Å range:
|
Calcium binding site 6 out of 6 in 6h6v
Go back to
Calcium binding site 6 out
of 6 in the Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn

Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 6 of Structure of the Ubid-Class Enzyme Hmff From Pelotomaculum Thermopropionicum in Complex with Fmn within 5.0Å range:
|
Reference:
K.A.P.Payne,
S.A.Marshall,
K.Fisher,
M.J.Cliff,
D.M.Cannas,
C.Yan,
D.J.Heyes,
D.A.Parker,
I.Larrosa,
D.Leys.
Enzymatic Carboxylation of 2-Furoic Acid Yields 2,5-Furandicarboxylic Acid (Fdca). Acs Catalysis V. 9 2854 2019.
ISSN: ESSN 2155-5435
PubMed: 31057985
DOI: 10.1021/ACSCATAL.8B04862
Page generated: Wed Jul 9 14:32:08 2025
ISSN: ESSN 2155-5435
PubMed: 31057985
DOI: 10.1021/ACSCATAL.8B04862
Last articles
Cl in 5JZSCl in 5K0E
Cl in 5JY3
Cl in 5JZN
Cl in 5JZB
Cl in 5JZL
Cl in 5JZ9
Cl in 5JZK
Cl in 5JY1
Cl in 5JYL