Calcium »
PDB 6mro-6n9d »
6n2b »
Calcium in PDB 6n2b: The Crystal Structure of Caldicellulosiruptor Kristjanssonii Tapirin C-Terminal Domain
Protein crystallography data
The structure of The Crystal Structure of Caldicellulosiruptor Kristjanssonii Tapirin C-Terminal Domain, PDB code: 6n2b
was solved by
P.M.Alahuhta,
V.V.Lunin,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 54.43 / 2.65 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 67.093, 98.317, 196.053, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 18.3 / 25.1 |
Calcium Binding Sites:
The binding sites of Calcium atom in the The Crystal Structure of Caldicellulosiruptor Kristjanssonii Tapirin C-Terminal Domain
(pdb code 6n2b). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 2 binding sites of Calcium where determined in the The Crystal Structure of Caldicellulosiruptor Kristjanssonii Tapirin C-Terminal Domain, PDB code: 6n2b:
Jump to Calcium binding site number: 1; 2;
In total 2 binding sites of Calcium where determined in the The Crystal Structure of Caldicellulosiruptor Kristjanssonii Tapirin C-Terminal Domain, PDB code: 6n2b:
Jump to Calcium binding site number: 1; 2;
Calcium binding site 1 out of 2 in 6n2b
Go back to
Calcium binding site 1 out
of 2 in the The Crystal Structure of Caldicellulosiruptor Kristjanssonii Tapirin C-Terminal Domain
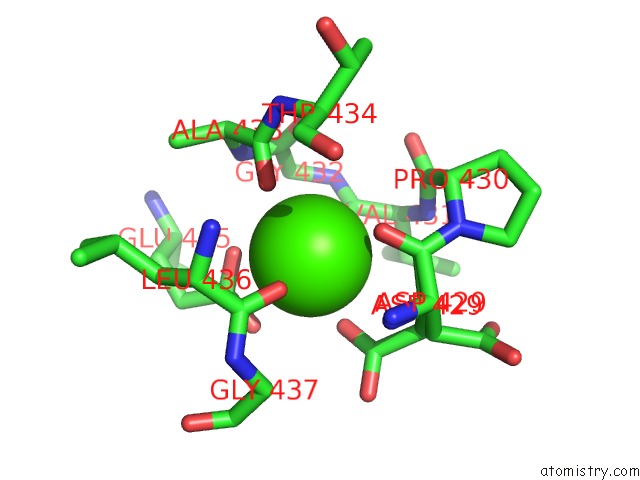
Mono view
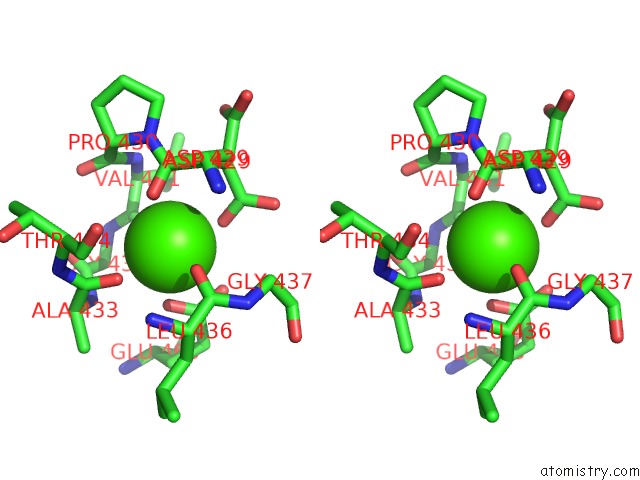
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of The Crystal Structure of Caldicellulosiruptor Kristjanssonii Tapirin C-Terminal Domain within 5.0Å range:
|
Calcium binding site 2 out of 2 in 6n2b
Go back to
Calcium binding site 2 out
of 2 in the The Crystal Structure of Caldicellulosiruptor Kristjanssonii Tapirin C-Terminal Domain
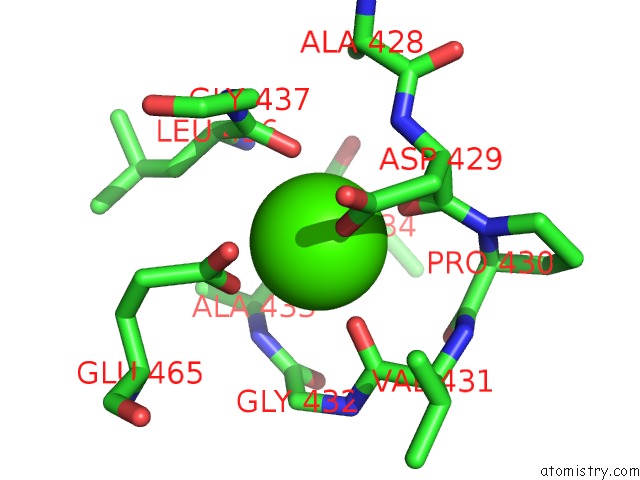
Mono view
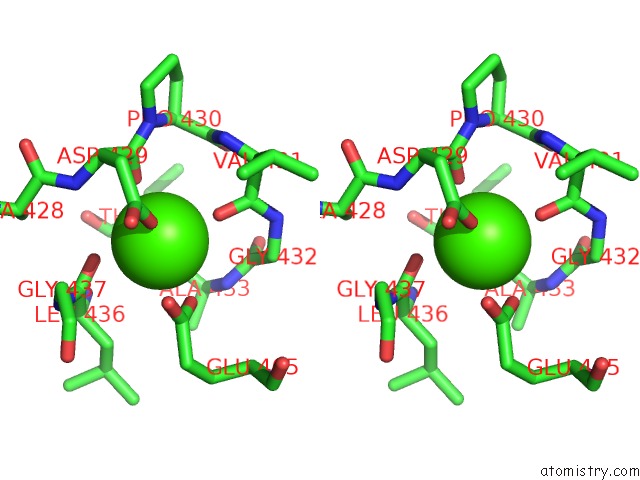
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of The Crystal Structure of Caldicellulosiruptor Kristjanssonii Tapirin C-Terminal Domain within 5.0Å range:
|
Reference:
L.L.Lee,
W.S.Hart,
V.V.Lunin,
M.Alahuhta,
Y.J.Bomble,
M.E.Himmel,
S.E.Blumer-Schuette,
M.W.W.Adams,
R.M.Kelly.
Comparative Biochemical and Structural Analysis of Novel Cellulose Binding Proteins (Tapirins) From Extremely Thermophiliccaldicellulosiruptorspecies. Appl. Environ. Microbiol. V. 85 2019.
ISSN: ESSN 1098-5336
PubMed: 30478233
DOI: 10.1128/AEM.01983-18
Page generated: Wed Jul 9 16:14:46 2025
ISSN: ESSN 1098-5336
PubMed: 30478233
DOI: 10.1128/AEM.01983-18
Last articles
Ca in 7KTACa in 7KWM
Ca in 7KW6
Ca in 7KT3
Ca in 7KSZ
Ca in 7KSS
Ca in 7KRY
Ca in 7KSE
Ca in 7KSF
Ca in 7KOX