Calcium »
PDB 8hcj-8ic1 »
8i5r »
Calcium in PDB 8i5r: Crystal Structure of TXGH116 D593N Acid/Base Mutant From Thermoanaerobacterium Xylanolyticum
Enzymatic activity of Crystal Structure of TXGH116 D593N Acid/Base Mutant From Thermoanaerobacterium Xylanolyticum
All present enzymatic activity of Crystal Structure of TXGH116 D593N Acid/Base Mutant From Thermoanaerobacterium Xylanolyticum:
3.2.1.21;
3.2.1.21;
Protein crystallography data
The structure of Crystal Structure of TXGH116 D593N Acid/Base Mutant From Thermoanaerobacterium Xylanolyticum, PDB code: 8i5r
was solved by
S.Pengthaisong,
J.R.Ketudat Cairns,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 35.00 / 1.65 |
| Space group | P 21 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 177.738, 54.246, 83.244, 90, 90, 90 |
| R / Rfree (%) | 14.8 / 17.6 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of TXGH116 D593N Acid/Base Mutant From Thermoanaerobacterium Xylanolyticum
(pdb code 8i5r). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total only one binding site of Calcium was determined in the Crystal Structure of TXGH116 D593N Acid/Base Mutant From Thermoanaerobacterium Xylanolyticum, PDB code: 8i5r:
In total only one binding site of Calcium was determined in the Crystal Structure of TXGH116 D593N Acid/Base Mutant From Thermoanaerobacterium Xylanolyticum, PDB code: 8i5r:
Calcium binding site 1 out of 1 in 8i5r
Go back to
Calcium binding site 1 out
of 1 in the Crystal Structure of TXGH116 D593N Acid/Base Mutant From Thermoanaerobacterium Xylanolyticum
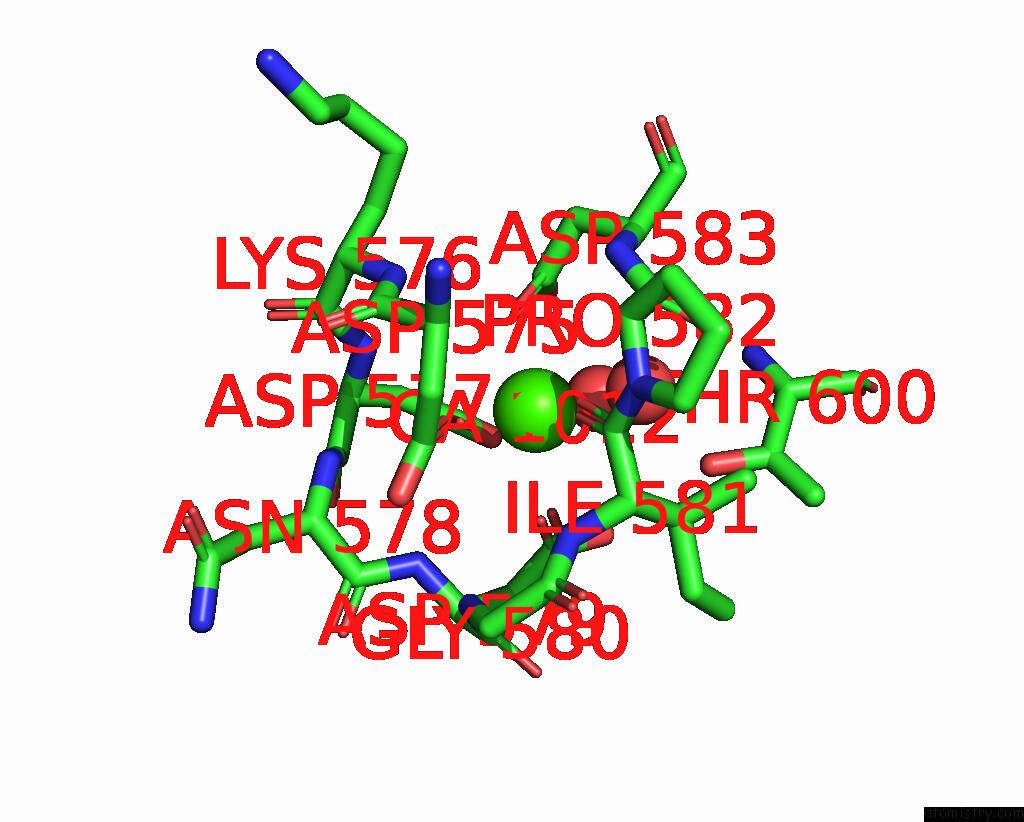
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of TXGH116 D593N Acid/Base Mutant From Thermoanaerobacterium Xylanolyticum within 5.0Å range:
|
Reference:
S.Pengthaisong,
B.Piniello,
G.J.Davies,
C.Rovira,
J.R.K.Cairns.
Reaction Mechanism of Glycoside Hydrolase Family 116 Utilizes Perpendicular Protonation Acs Catalysis 5850 2023.
ISSN: ESSN 2155-5435
DOI: 10.1021/ACSCATAL.3C00620
Page generated: Thu Jul 10 05:11:48 2025
ISSN: ESSN 2155-5435
DOI: 10.1021/ACSCATAL.3C00620
Last articles
Cl in 5QHHCl in 5QHG
Cl in 5QH1
Cl in 5QGZ
Cl in 5QGY
Cl in 5QGX
Cl in 5QFZ
Cl in 5QGV
Cl in 5QGS
Cl in 5QFJ