Calcium »
PDB 8q2a-8qx0 »
8qwx »
Calcium in PDB 8qwx: Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms
Enzymatic activity of Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms
All present enzymatic activity of Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms:
1.11.1.13;
1.11.1.13;
Protein crystallography data
The structure of Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms, PDB code: 8qwx
was solved by
E.Santillana,
A.Romero,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.78 / 1.60 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 62.623, 39.63, 63.493, 90, 101.23, 90 |
| R / Rfree (%) | 15.1 / 18.6 |
Other elements in 8qwx:
The structure of Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms also contains other interesting chemical elements:
| Iron | (Fe) | 1 atom |
Calcium Binding Sites:
The binding sites of Calcium atom in the Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms
(pdb code 8qwx). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 2 binding sites of Calcium where determined in the Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms, PDB code: 8qwx:
Jump to Calcium binding site number: 1; 2;
In total 2 binding sites of Calcium where determined in the Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms, PDB code: 8qwx:
Jump to Calcium binding site number: 1; 2;
Calcium binding site 1 out of 2 in 8qwx
Go back to
Calcium binding site 1 out
of 2 in the Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms
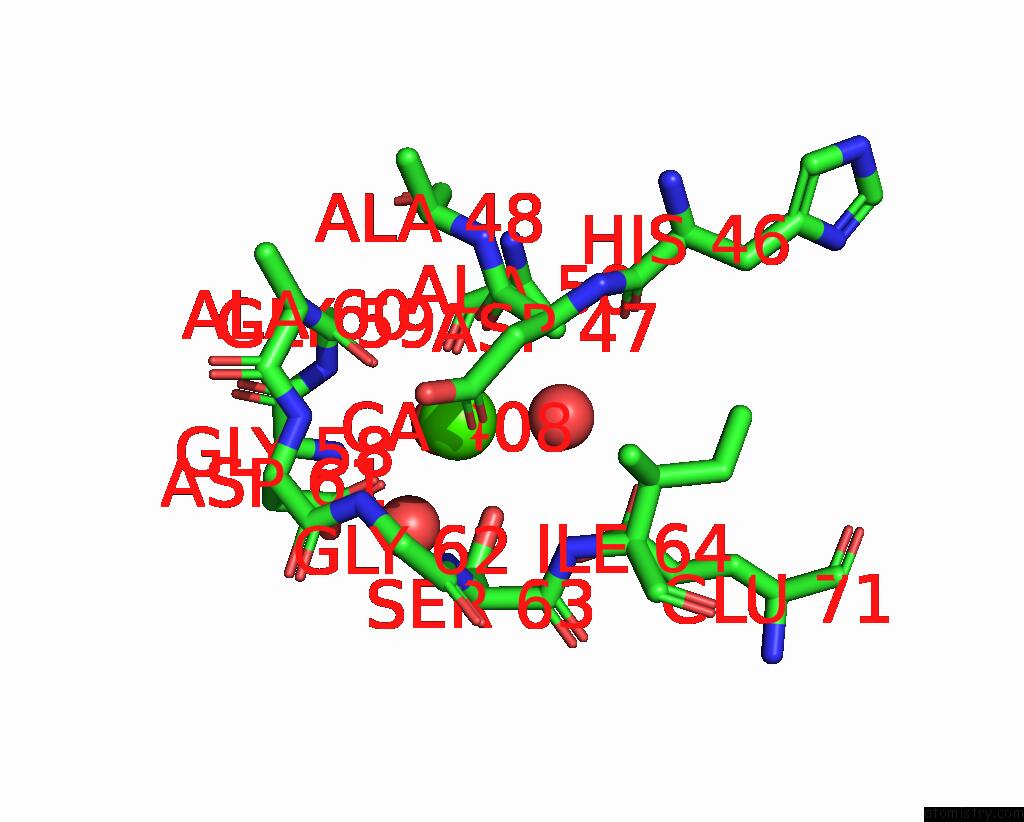
Mono view
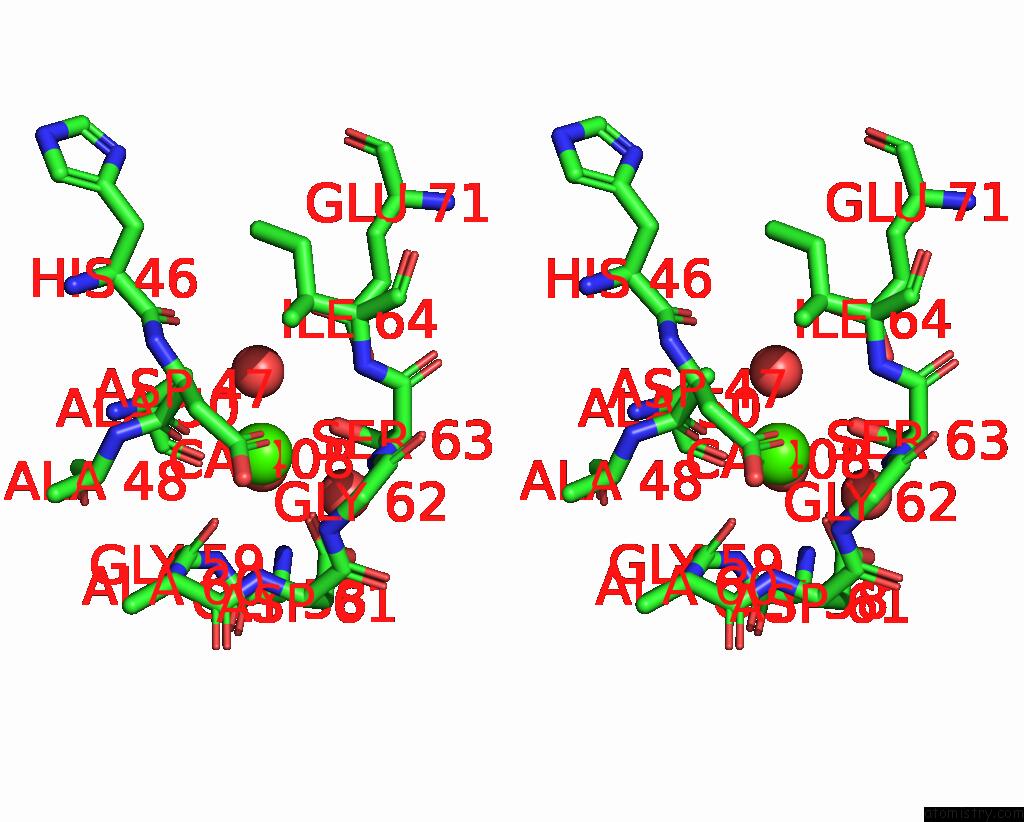
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms within 5.0Å range:
|
Calcium binding site 2 out of 2 in 8qwx
Go back to
Calcium binding site 2 out
of 2 in the Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms
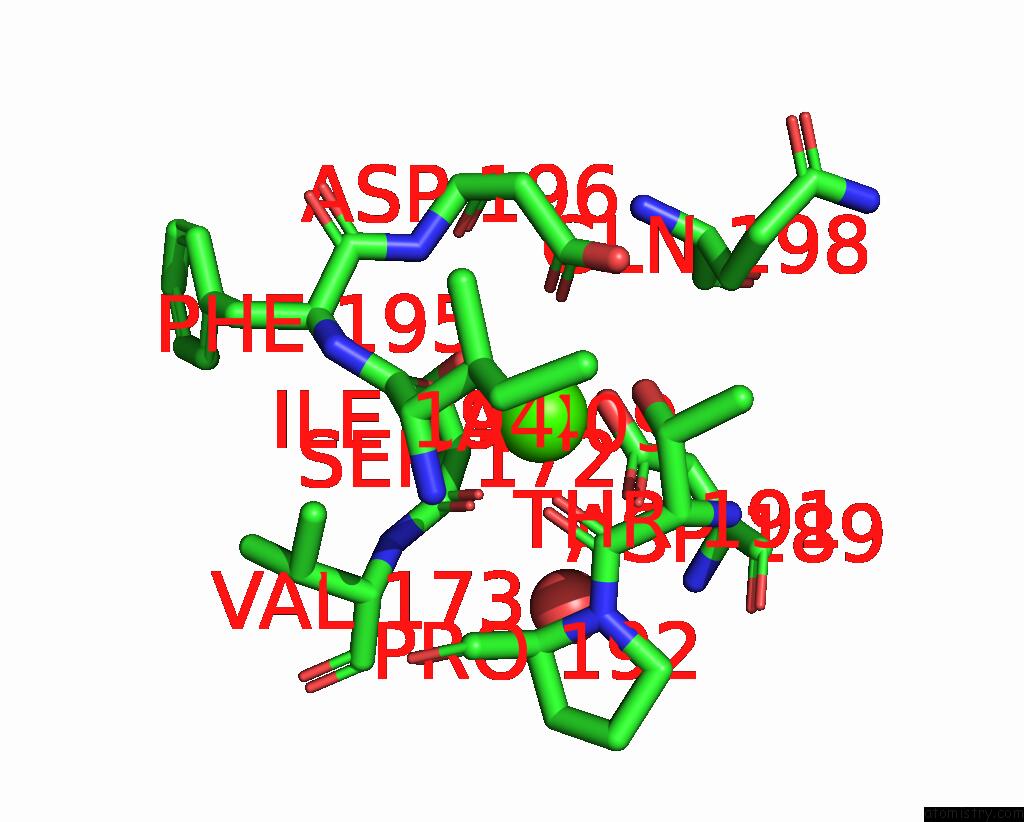
Mono view
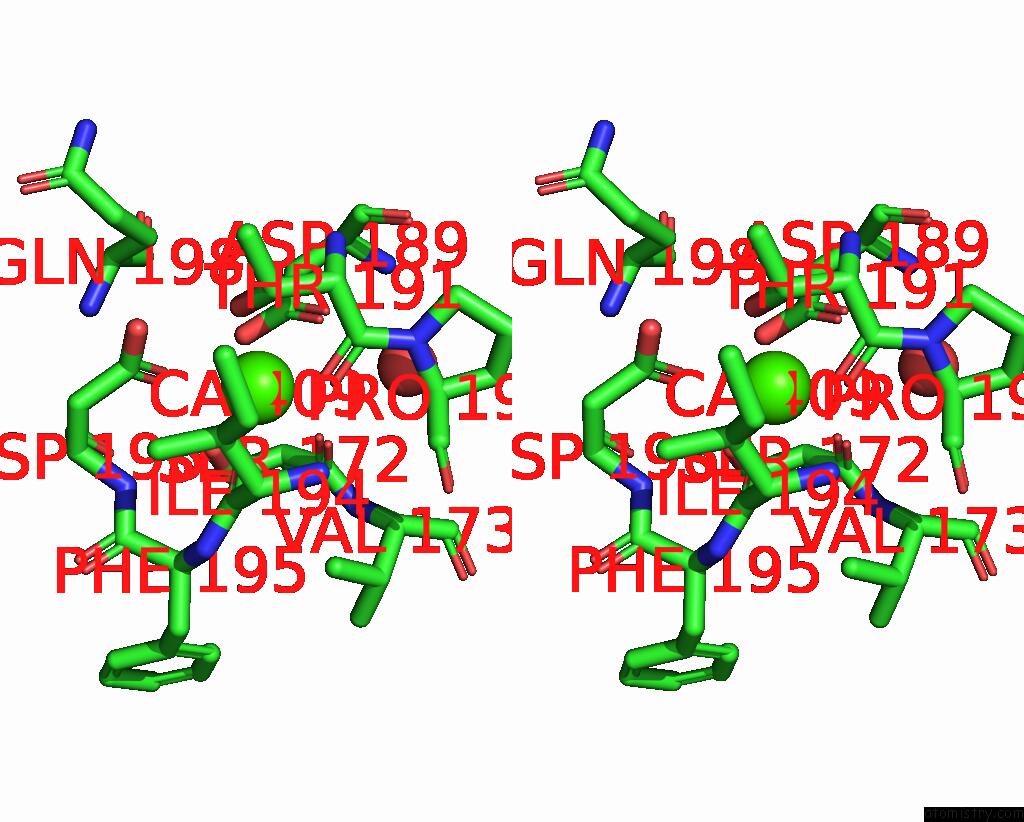
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Ligninolytic Manganese Peroxidase Ape-MNP1 From Agaricales Mushrooms within 5.0Å range:
|
Reference:
M.I.Sanchez-Ruiz,
E.Santillana,
D.Linde,
A.Romero,
A.T.Martinez,
F.J.Ruiz-Duenas.
Structure-Function Characterization of Two Enzymes From Novel Subfamilies of Manganese Peroxidases Secreted By the Lignocellulose-Degrading Agaricales Fungi Agrocybe Pediades and Cyathus Striatus. Biotechnol Biofuels Bioprod V. 17 74 2024.
ISSN: ISSN 2731-3654
PubMed: 38824538
DOI: 10.1186/S13068-024-02517-1
Page generated: Thu Jul 10 06:40:35 2025
ISSN: ISSN 2731-3654
PubMed: 38824538
DOI: 10.1186/S13068-024-02517-1
Last articles
Cl in 5RAVCl in 5RAU
Cl in 5RAS
Cl in 5RAR
Cl in 5RAQ
Cl in 5RAP
Cl in 5RAO
Cl in 5RAN
Cl in 5RAJ
Cl in 5RAM