Calcium »
PDB 8tr2-8uwm »
8uf5 »
Calcium in PDB 8uf5: Catalytic Domain of Gtfb in Complex with Inhibitor G43
Enzymatic activity of Catalytic Domain of Gtfb in Complex with Inhibitor G43
All present enzymatic activity of Catalytic Domain of Gtfb in Complex with Inhibitor G43:
2.4.1.5;
2.4.1.5;
Protein crystallography data
The structure of Catalytic Domain of Gtfb in Complex with Inhibitor G43, PDB code: 8uf5
was solved by
N.Schormann,
C.Deivanayagam,
S.Velu,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 56.35 / 2.50 |
| Space group | P 43 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 149.777, 149.777, 304.065, 90, 90, 90 |
| R / Rfree (%) | 19.9 / 23 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Catalytic Domain of Gtfb in Complex with Inhibitor G43
(pdb code 8uf5). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 2 binding sites of Calcium where determined in the Catalytic Domain of Gtfb in Complex with Inhibitor G43, PDB code: 8uf5:
Jump to Calcium binding site number: 1; 2;
In total 2 binding sites of Calcium where determined in the Catalytic Domain of Gtfb in Complex with Inhibitor G43, PDB code: 8uf5:
Jump to Calcium binding site number: 1; 2;
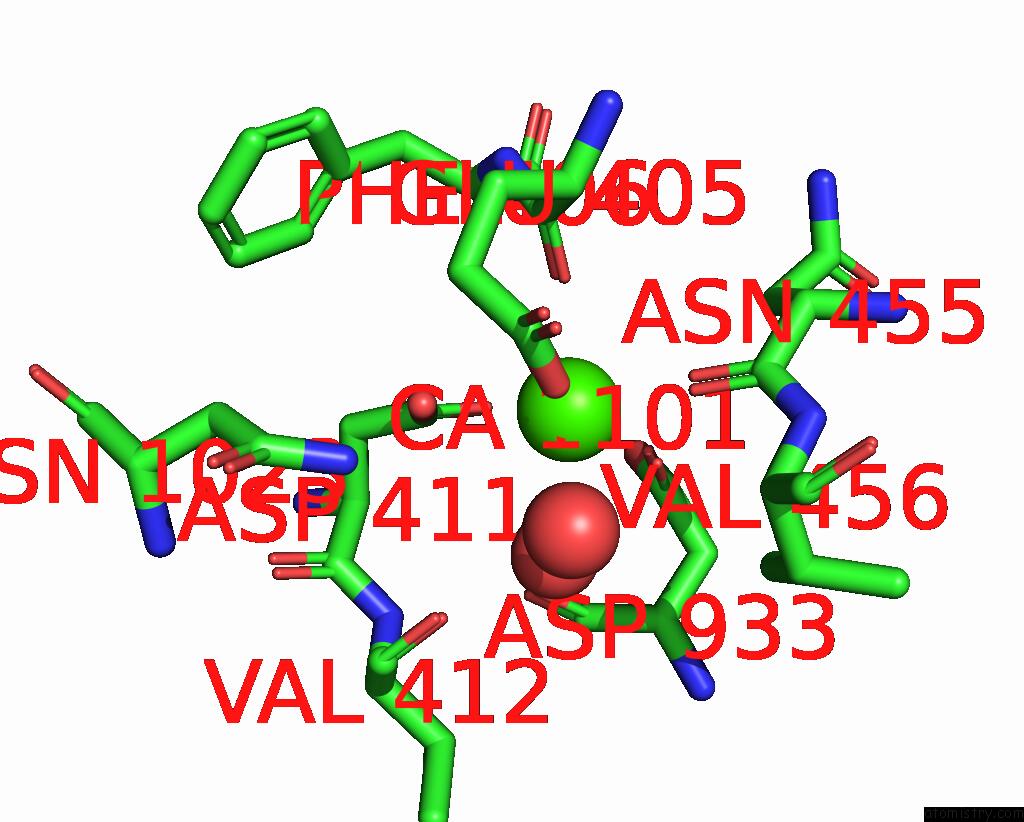
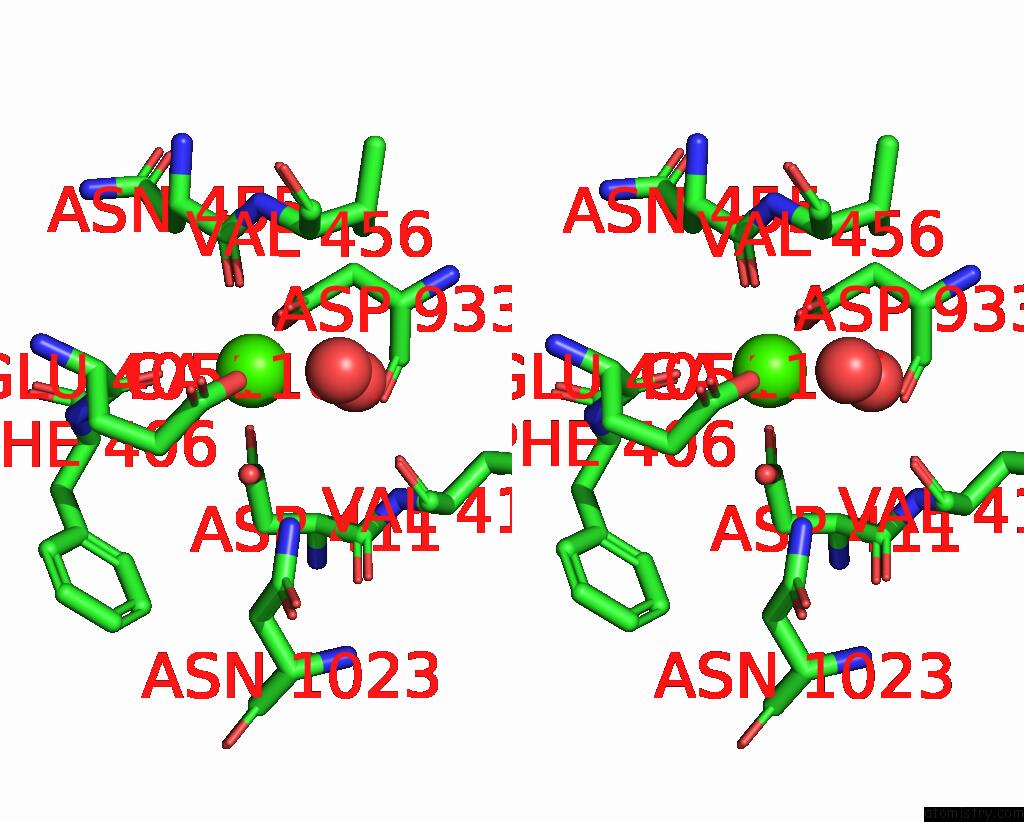
Calcium binding site 1 out of 2 in 8uf5
Go back to
Calcium binding site 1 out
of 2 in the Catalytic Domain of Gtfb in Complex with Inhibitor G43
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Catalytic Domain of Gtfb in Complex with Inhibitor G43 within 5.0Å range:
|
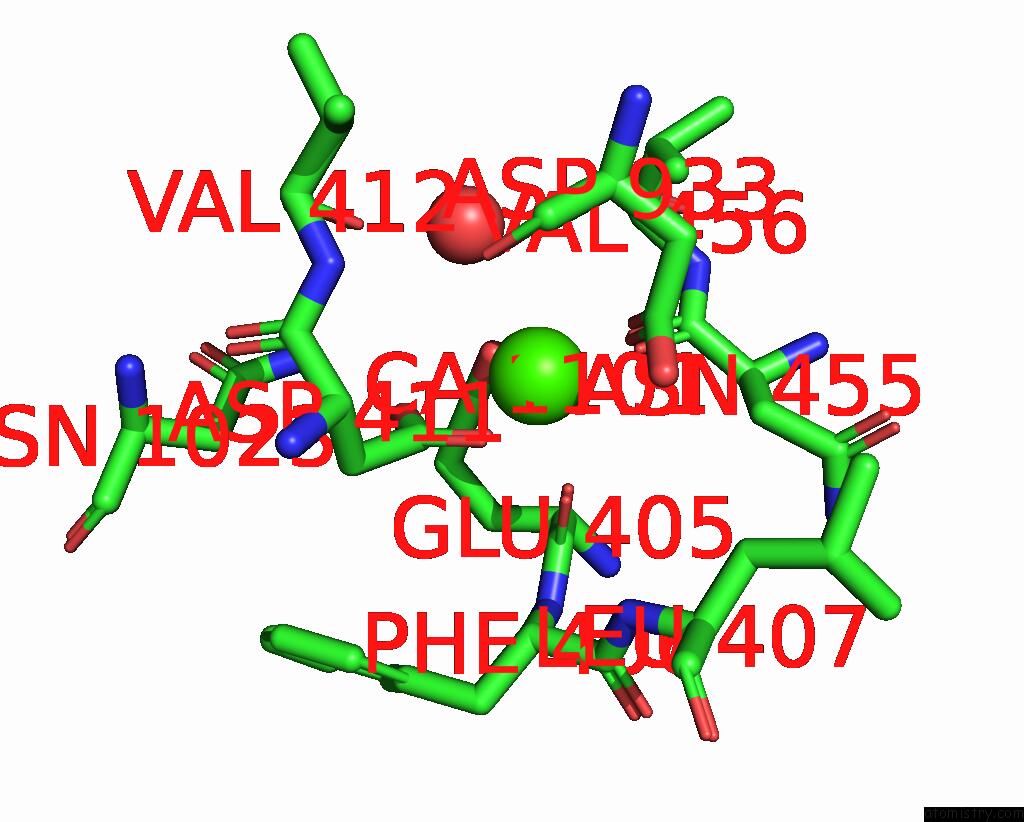
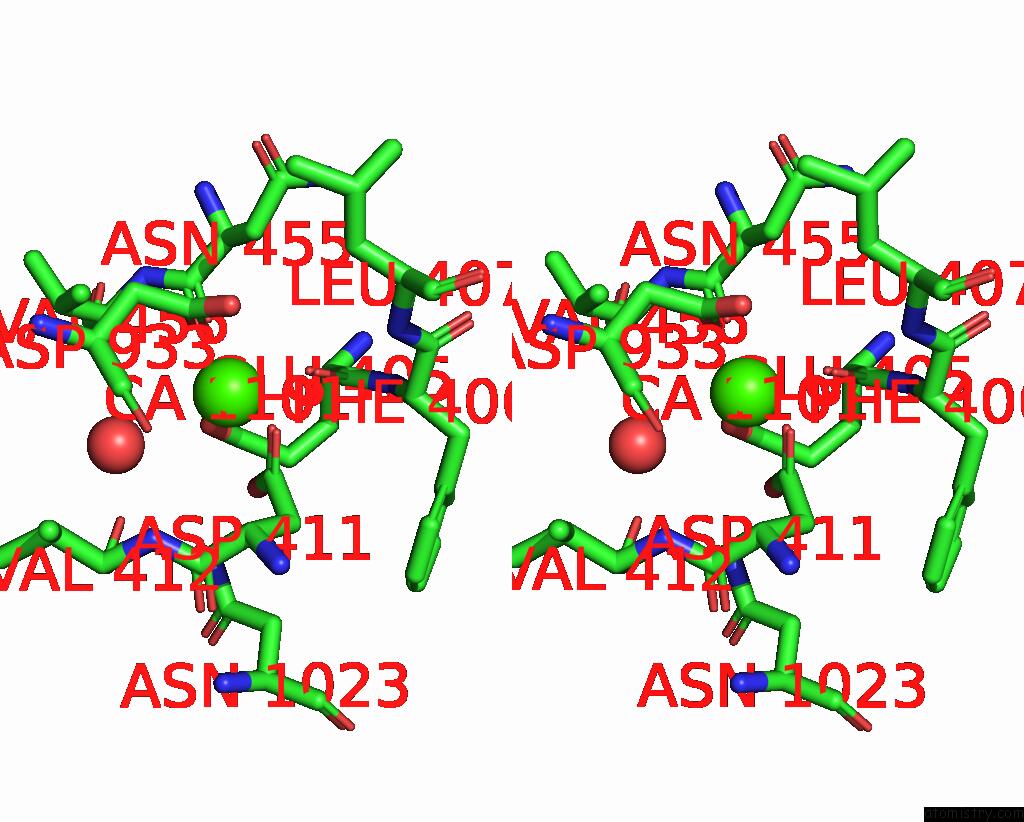
Calcium binding site 2 out of 2 in 8uf5
Go back to
Calcium binding site 2 out
of 2 in the Catalytic Domain of Gtfb in Complex with Inhibitor G43
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Catalytic Domain of Gtfb in Complex with Inhibitor G43 within 5.0Å range:
|
Reference:
Q.Zhang,
B.Nijampatnam,
Z.Hua,
T.Nguyen,
J.Zou,
X.Cai,
S.M.Michalek,
S.E.Velu,
H.Wu.
Structure-Based Discovery of Small Molecule Inhibitors of Cariogenic Virulence. Sci Rep V. 7 5974 2017.
ISSN: ESSN 2045-2322
PubMed: 28729722
DOI: 10.1177/00220345580370060901
Page generated: Thu Jul 10 07:38:36 2025
ISSN: ESSN 2045-2322
PubMed: 28729722
DOI: 10.1177/00220345580370060901
Last articles
Cl in 5QI6Cl in 5QI5
Cl in 5QI7
Cl in 5QI4
Cl in 5QI3
Cl in 5QI2
Cl in 5QI1
Cl in 5QI0
Cl in 5QHZ
Cl in 5QHW