Calcium »
PDB 1b85-1bjj »
1b8l »
Calcium in PDB 1b8l: Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin
Protein crystallography data
The structure of Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin, PDB code: 1b8l
was solved by
M.S.Cates,
M.B.Berry,
E.Ho,
Q.Li,
J.D.Potter,
G.N.Phillips Jr.,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 40.00 / 1.70 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 34.511, 37.298, 37.067, 90.00, 113.12, 90.00 |
| R / Rfree (%) | 16.8 / 22.7 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin
(pdb code 1b8l). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total only one binding site of Calcium was determined in the Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin, PDB code: 1b8l:
In total only one binding site of Calcium was determined in the Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin, PDB code: 1b8l:
Calcium binding site 1 out of 1 in 1b8l
Go back to
Calcium binding site 1 out
of 1 in the Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin
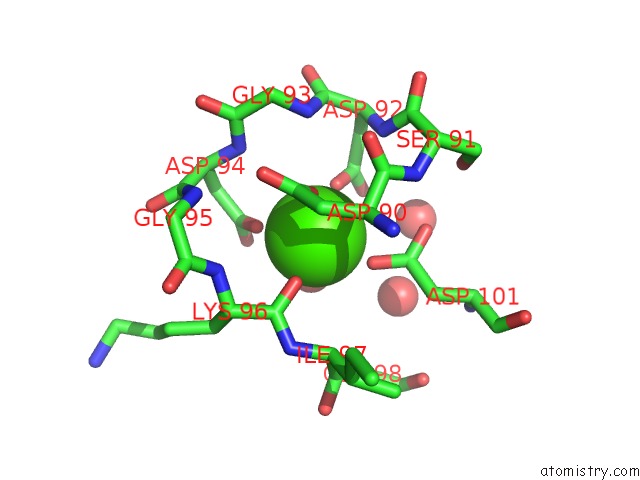
Mono view
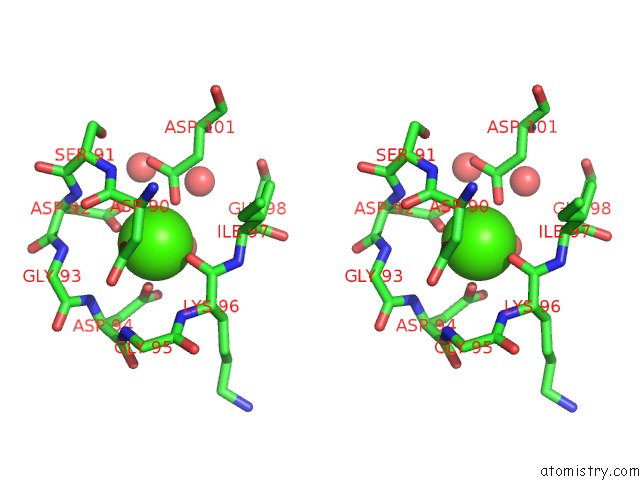
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Calcium-Bound D51A/E101D/F102W Triple Mutant of Beta Carp Parvalbumin within 5.0Å range:
|
Reference:
M.S.Cates,
M.B.Berry,
E.L.Ho,
Q.Li,
J.D.Potter,
G.N.Phillips Jr..
Metal-Ion Affinity and Specificity in Ef-Hand Proteins: Coordination Geometry and Domain Plasticity in Parvalbumin. Structure Fold.Des. V. 7 1269 1999.
ISSN: ISSN 0969-2126
PubMed: 10545326
DOI: 10.1016/S0969-2126(00)80060-X
Page generated: Mon Jul 7 13:36:48 2025
ISSN: ISSN 0969-2126
PubMed: 10545326
DOI: 10.1016/S0969-2126(00)80060-X
Last articles
Mg in 3X0EMg in 3X1L
Mg in 3X1D
Mg in 3WZY
Mg in 3WZX
Mg in 3WYM
Mg in 3WZW
Mg in 3WZV
Mg in 3WYL
Mg in 3WVL