Calcium »
PDB 3v0a-3vl2 »
3veq »
Calcium in PDB 3veq: A Binary Complex Betwwen Bovine Pancreatic Trypsin and A Engineered Mutant Trypsin Inhibitor
Enzymatic activity of A Binary Complex Betwwen Bovine Pancreatic Trypsin and A Engineered Mutant Trypsin Inhibitor
All present enzymatic activity of A Binary Complex Betwwen Bovine Pancreatic Trypsin and A Engineered Mutant Trypsin Inhibitor:
3.4.21.4;
3.4.21.4;
Protein crystallography data
The structure of A Binary Complex Betwwen Bovine Pancreatic Trypsin and A Engineered Mutant Trypsin Inhibitor, PDB code: 3veq
was solved by
U.Sen,
S.Majumder,
S.Khamrui,
J.Dasgupta,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.91 / 2.25 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 122.997, 39.826, 76.783, 90.00, 119.65, 90.00 |
| R / Rfree (%) | 21.8 / 27.4 |
Calcium Binding Sites:
The binding sites of Calcium atom in the A Binary Complex Betwwen Bovine Pancreatic Trypsin and A Engineered Mutant Trypsin Inhibitor
(pdb code 3veq). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total only one binding site of Calcium was determined in the A Binary Complex Betwwen Bovine Pancreatic Trypsin and A Engineered Mutant Trypsin Inhibitor, PDB code: 3veq:
In total only one binding site of Calcium was determined in the A Binary Complex Betwwen Bovine Pancreatic Trypsin and A Engineered Mutant Trypsin Inhibitor, PDB code: 3veq:
Calcium binding site 1 out of 1 in 3veq
Go back to
Calcium binding site 1 out
of 1 in the A Binary Complex Betwwen Bovine Pancreatic Trypsin and A Engineered Mutant Trypsin Inhibitor
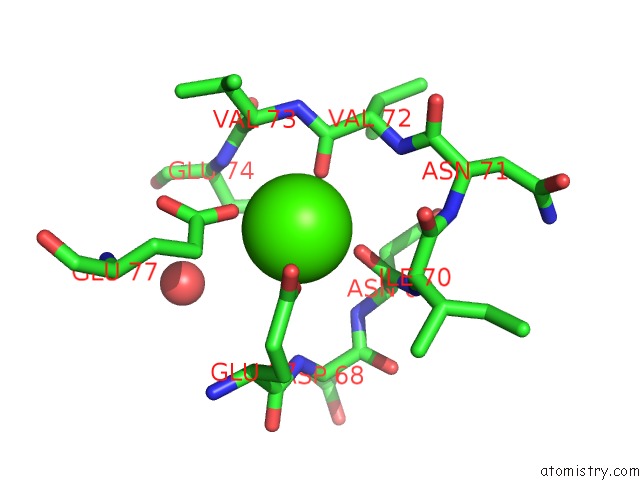
Mono view
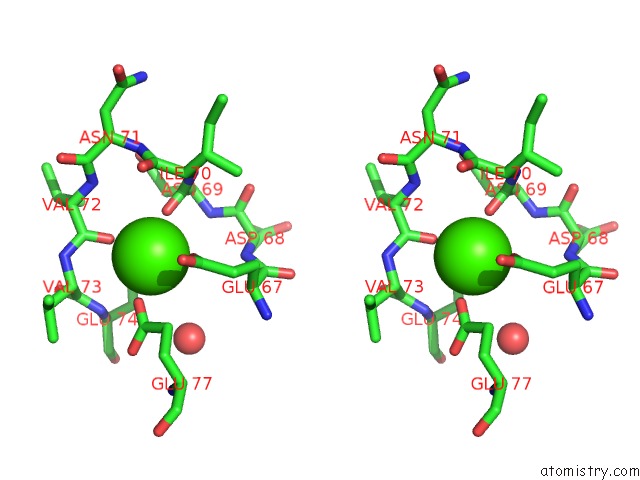
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of A Binary Complex Betwwen Bovine Pancreatic Trypsin and A Engineered Mutant Trypsin Inhibitor within 5.0Å range:
|
Reference:
S.Majumder,
S.Khamrui,
J.Dasgupta,
J.K.Dattagupta,
U.Sen.
Role of Remote Scaffolding Residues in the Inhibitory Loop Pre-Organization, Flexibility, Rigidification and Enzyme Inhibition of Serine Protease Inhibitors Biochim.Biophys.Acta V.1824 882 2012.
ISSN: ISSN 0006-3002
PubMed: 22709512
DOI: 10.1016/J.BBAPAP.2012.04.009
Page generated: Tue Jul 8 17:34:05 2025
ISSN: ISSN 0006-3002
PubMed: 22709512
DOI: 10.1016/J.BBAPAP.2012.04.009
Last articles
Mg in 5G0RMg in 5G5V
Mg in 5G3T
Mg in 5G5T
Mg in 5G5S
Mg in 5G4A
Mg in 5G57
Mg in 5G50
Mg in 5G41
Mg in 5G3Z