Calcium »
PDB 4y9p-4yty »
4yic »
Calcium in PDB 4yic: Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid
Protein crystallography data
The structure of Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid, PDB code: 4yic
was solved by
M.W.Vetting,
N.F.Al Obaidi,
R.Toro,
L.L.Morisco,
J.Benach,
J.Koss,
S.R.Wasserman,
J.D.Attonito,
A.Scott Glenn,
S.Chamala,
S.Chowdhury,
J.Lafleur,
J.Love,
R.D.Seidel,
K.L.Whalen,
J.A.Gerlt,
S.C.Almo,
Enzymefunction Initiative (Efi),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 25.56 / 1.60 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 64.681, 86.141, 72.339, 90.00, 100.86, 90.00 |
| R / Rfree (%) | 14.3 / 17.3 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid
(pdb code 4yic). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 2 binding sites of Calcium where determined in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid, PDB code: 4yic:
Jump to Calcium binding site number: 1; 2;
In total 2 binding sites of Calcium where determined in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid, PDB code: 4yic:
Jump to Calcium binding site number: 1; 2;
Calcium binding site 1 out of 2 in 4yic
Go back to
Calcium binding site 1 out
of 2 in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid
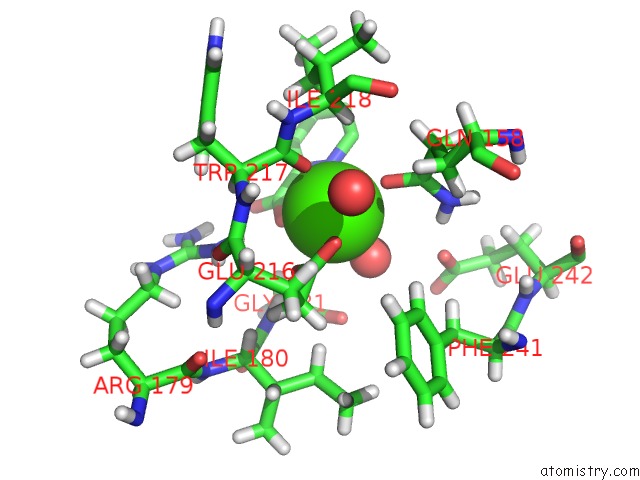
Mono view
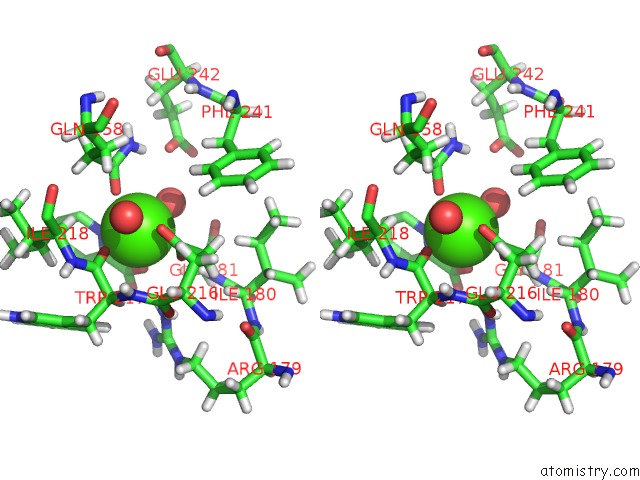
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid within 5.0Å range:
|
Calcium binding site 2 out of 2 in 4yic
Go back to
Calcium binding site 2 out
of 2 in the Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid
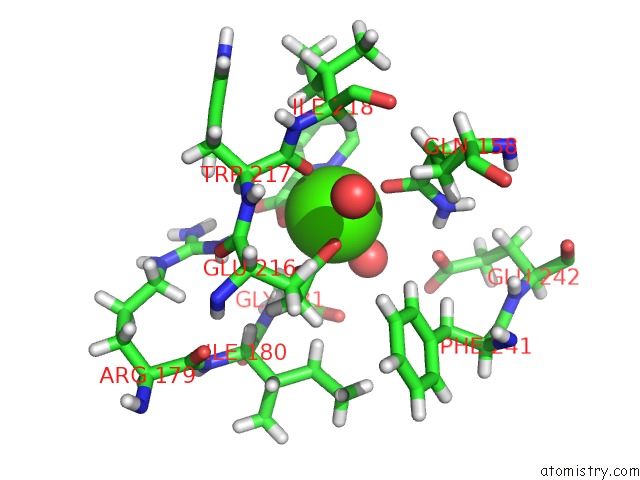
Mono view
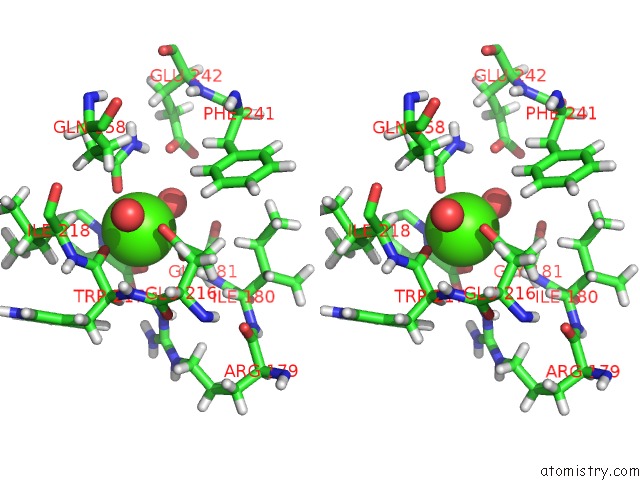
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi- 500035) with Bound Picolinic Acid within 5.0Å range:
|
Reference:
M.W.Vetting,
N.F.Al Obaidi,
R.Toro,
L.L.Morisco,
J.Benach,
J.Koss,
S.R.Wasserman,
J.D.Attonito,
A.Scott Glenn,
S.Chamala,
S.Chowdhury,
J.Lafleur,
J.Love,
R.D.Seidel,
K.L.Whalen,
J.A.Gerlt,
S.C.Almo,
Enzyme Function Initiative (Efi).
Crystal Structure of A Trap Transporter Solute Binding Protein (IPR025997) From Bordetella Bronchiseptica RB50 (BB0280, Target Efi-500035) with Bound Picolinic Acid To Be Published.
Page generated: Wed Jul 9 03:12:05 2025
Last articles
I in 3WN5I in 3WYX
I in 3WGW
I in 3WD6
I in 3WB5
I in 3W31
I in 3WB4
I in 3W1N
I in 3W0F
I in 3W2Z