Calcium »
PDB 3hjb-3hz3 »
3hkr »
Calcium in PDB 3hkr: Crystal Structure of Glutathione Transferase Pi Y108V Mutant
Enzymatic activity of Crystal Structure of Glutathione Transferase Pi Y108V Mutant
All present enzymatic activity of Crystal Structure of Glutathione Transferase Pi Y108V Mutant:
2.5.1.18;
2.5.1.18;
Protein crystallography data
The structure of Crystal Structure of Glutathione Transferase Pi Y108V Mutant, PDB code: 3hkr
was solved by
L.J.Parker,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 42.11 / 1.80 |
| Space group | C 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 77.100, 90.300, 68.900, 90.00, 98.20, 90.00 |
| R / Rfree (%) | 17 / 21 |
Calcium Binding Sites:
The binding sites of Calcium atom in the Crystal Structure of Glutathione Transferase Pi Y108V Mutant
(pdb code 3hkr). This binding sites where shown within
5.0 Angstroms radius around Calcium atom.
In total 5 binding sites of Calcium where determined in the Crystal Structure of Glutathione Transferase Pi Y108V Mutant, PDB code: 3hkr:
Jump to Calcium binding site number: 1; 2; 3; 4; 5;
In total 5 binding sites of Calcium where determined in the Crystal Structure of Glutathione Transferase Pi Y108V Mutant, PDB code: 3hkr:
Jump to Calcium binding site number: 1; 2; 3; 4; 5;
Calcium binding site 1 out of 5 in 3hkr
Go back to
Calcium binding site 1 out
of 5 in the Crystal Structure of Glutathione Transferase Pi Y108V Mutant
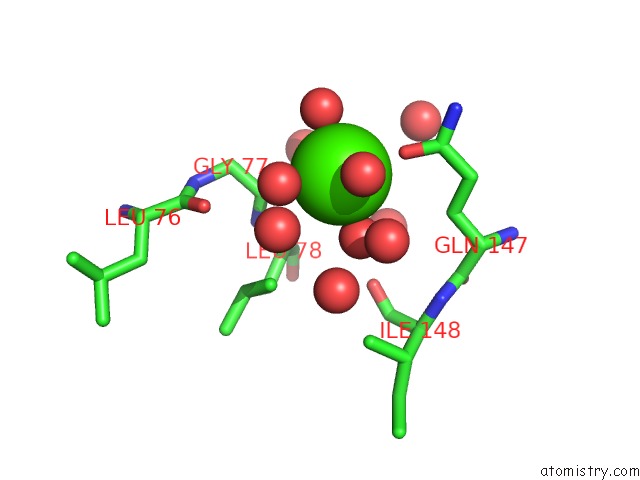
Mono view
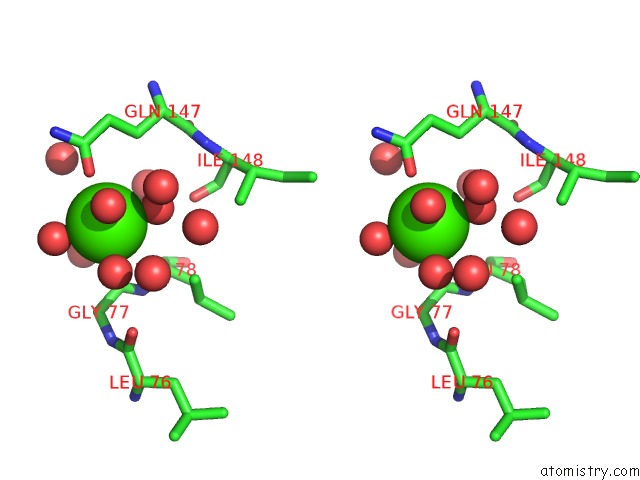
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 1 of Crystal Structure of Glutathione Transferase Pi Y108V Mutant within 5.0Å range:
|
Calcium binding site 2 out of 5 in 3hkr
Go back to
Calcium binding site 2 out
of 5 in the Crystal Structure of Glutathione Transferase Pi Y108V Mutant
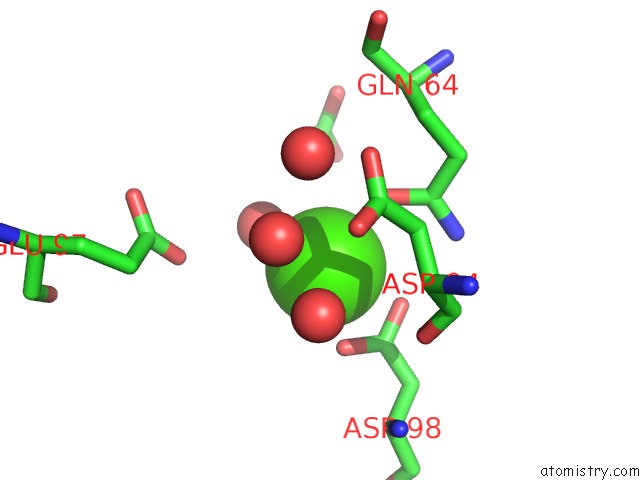
Mono view
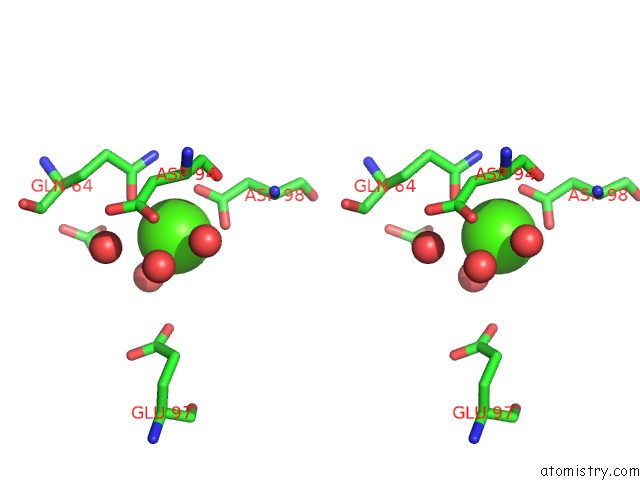
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 2 of Crystal Structure of Glutathione Transferase Pi Y108V Mutant within 5.0Å range:
|
Calcium binding site 3 out of 5 in 3hkr
Go back to
Calcium binding site 3 out
of 5 in the Crystal Structure of Glutathione Transferase Pi Y108V Mutant
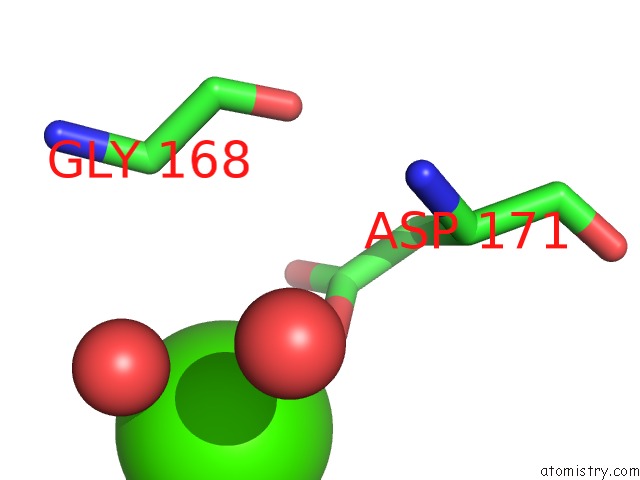
Mono view
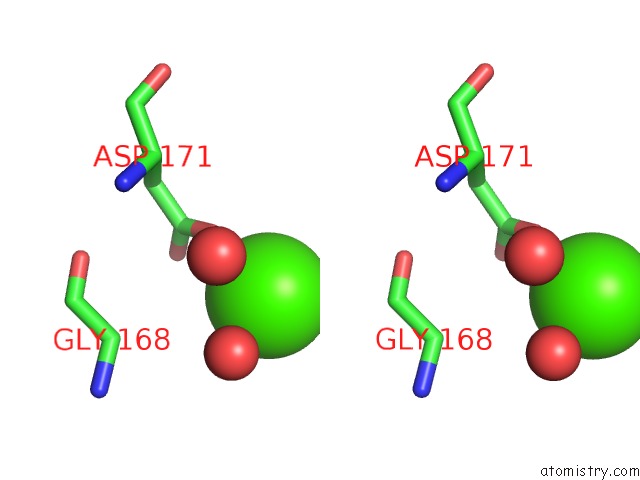
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 3 of Crystal Structure of Glutathione Transferase Pi Y108V Mutant within 5.0Å range:
|
Calcium binding site 4 out of 5 in 3hkr
Go back to
Calcium binding site 4 out
of 5 in the Crystal Structure of Glutathione Transferase Pi Y108V Mutant
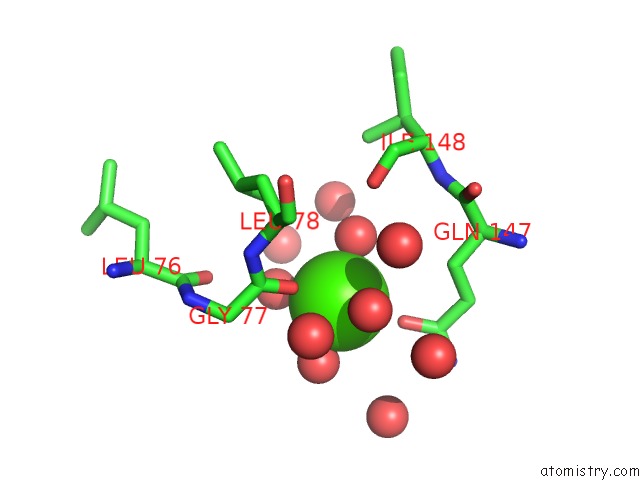
Mono view
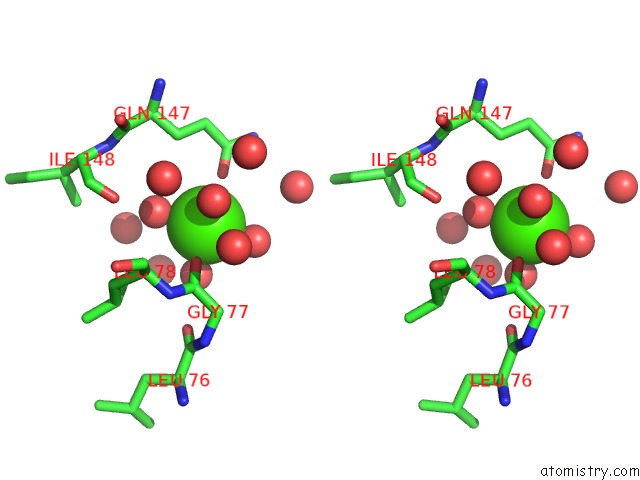
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 4 of Crystal Structure of Glutathione Transferase Pi Y108V Mutant within 5.0Å range:
|
Calcium binding site 5 out of 5 in 3hkr
Go back to
Calcium binding site 5 out
of 5 in the Crystal Structure of Glutathione Transferase Pi Y108V Mutant
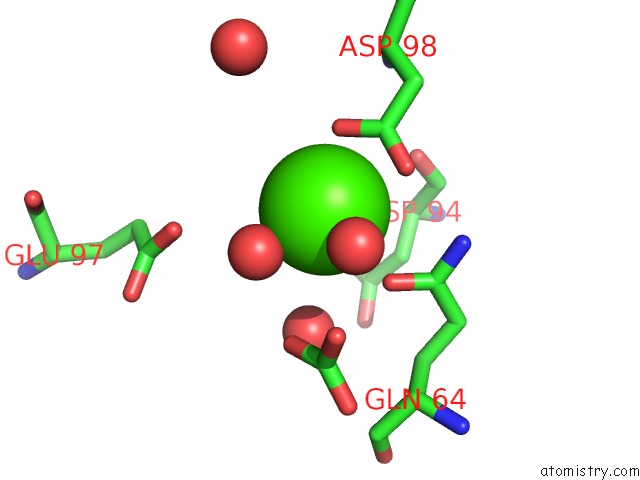
Mono view
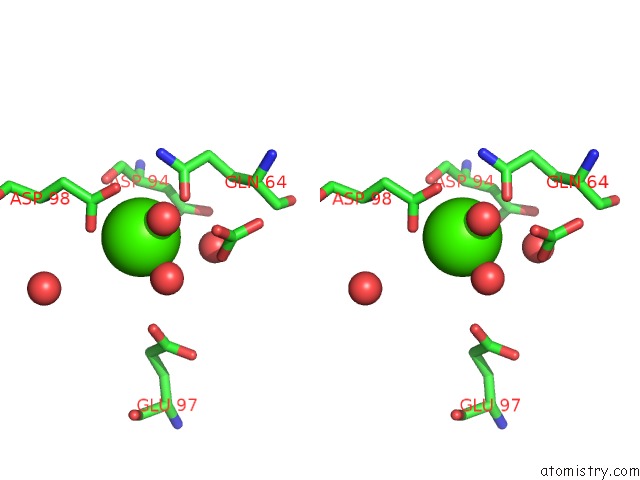
Stereo pair view

Mono view

Stereo pair view
A full contact list of Calcium with other atoms in the Ca binding
site number 5 of Crystal Structure of Glutathione Transferase Pi Y108V Mutant within 5.0Å range:
|
Reference:
I.Quesada-Soriano,
L.J.Parker,
A.Primavera,
J.M.Casas-Solvas,
A.Vargas-Berenguel,
C.Baron,
C.J.Morton,
A.P.Mazzetti,
M.Lo Bello,
M.W.Parker,
L.Garcia-Fuentes.
Influence of the H-Site Residue 108 on Human Glutathione Transferase P1-1 Ligand Binding: Structure-Thermodynamic Relationships and Thermal Stability. Protein Sci. V. 18 2454 2009.
ISSN: ISSN 0961-8368
PubMed: 19780048
DOI: 10.1002/PRO.253
Page generated: Tue Jul 8 13:01:01 2025
ISSN: ISSN 0961-8368
PubMed: 19780048
DOI: 10.1002/PRO.253
Last articles
Ca in 3I08Ca in 3I46
Ca in 3I4P
Ca in 3I4I
Ca in 3I3S
Ca in 3I37
Ca in 3I34
Ca in 3I30
Ca in 3I2Y
Ca in 3I29